Case Report
Volume 3, Issue 6
Hemophagocytic Lymphohistiocytosis Triggered by COVID-19: A Case Report and Literature Review
Yuexia Zhang1,2; Qian Zhang1; Xuan Wang3; Rui Song1; Jiaofeng Bai1; Ruirui Zheng1; Zhichen Zhang1; Bianli Lian1; Yaozhu Pan1*
1Department of Hematology, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou, China.
2Graduate School of Qinghai University, Xining 810000, Qinghai Province, China.
3Department of Endocrinology, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou 730050, Gansu Province, China.
Corresponding Author :
Yaozhu Pan
Email: 18794862698@163.com
Received : May 17, 2024 Accepted : Jun 19, 2024 Published : Jun 26, 2024 Archived : www.meddiscoveries.org
Citation: Zhang Y, Zhang Q, Wang X, Song R, Pan Y, et al. Hemophagocytic Lymphohistiocytosis Triggered by COVID-19: A Case Report and Literature Review. Med Discoveries. 2024; 3(6): 1176.
Copyright: © 2024 Pan Y. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Hemophagocytic Lymphohistiocytosis (HLH) is a rare life-threatening inflammatory response syndrome associated with immune deregulation, which is prone to misdiagnosis due to the lack of specificity of clinical symptoms, leading to poor prognosis. COVID-19 is an acute respiratory disease with high infectiousness caused by the novel coronavirus SARS-CoV-2. Currently, HLH induced by COVID-19 is rare, increasing the complexity of clinical diagnosis. In our hematology department, we encountered a case of HLH triggered by COVID-19 with severe condition. After the diagnosis was clarified, the patient received the HLH-2004 treatment programme immediately. The patient is currently in complete remission. Given the rarity of the reported cases, we would like to share this case to provide clinicians with insights for the diagnosis and treatment of the condition.
Keywords: COVID-19; SARS-CoV-2; Hemophagocytic lymphohistiocytosis.
Introduction
HLH is a highly aggressive and life-threatening condition characterized by excessive activation of the immune system, which leads to multi-organ dysfunction. It was first described by Farquhar and Claireaux in 1952 [1]. Due to its high mortality rate, prompt diagnosis is of utmost importance. Since the SARSCoV-2 epidemic, COVID-19 has been identified as an important triggers for secondary HLH [2]. Here, we report a case of secondary HLH induced by COVID-19.
Case presentation
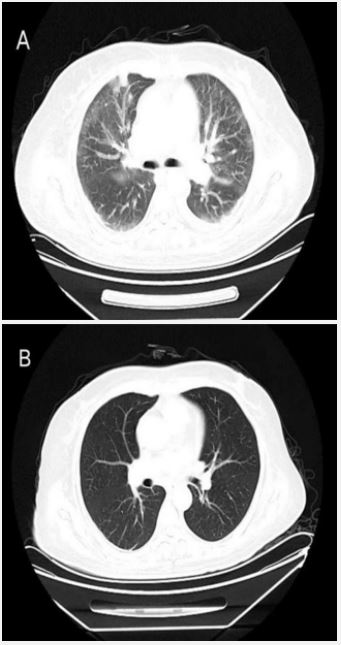
A 56-year-old female patient developed fever 8 days ago without obvious cause, she presented with a persistent high fever (up to 39.5°C). Treatment with antibiotics and nonsteroidal anti-inflammatory drugs was ineffective at the local hospital, Complete Blood Count (CBC) showed pancytopenia, so the patient was transferred to our hospital. The initial evaluation on admission showed pancytopenia (leucocyte count, 1.88×109 /L, neutrophil count, 0.77×109 /L, hemoglobin, 90 g/L, platelet, 28×109 /L), elevated triglyceride (5.62 mmol/L), decreased fibrinogen (1.25 g/L), and increased transaminase (aspartate aminotransferase 501U/L, alanine aminotransferase 111 U/L) and lactate dehydrogenase (3462 U/L) levels. COVID-19 nasal swab Polymerase Chain Reaction (PCR) test was positive. The chest Computed Tomography (CT) scan indicated interstitial pulmonary oedema, viral pneumonia (Figure 1). The patient received nirmatrelvir (300 mg,qd) and ritonavir (100 mg,qd). However, after 5 days of treatment, the patient again experienced fever, further examination suggested elevated ferritin levels (>40000.0 ug/L), decreased NK cell activity (3.2%), increased levels of sIL-2R/sCD25 (2869 U/mL), Epstein-Barr Virus (EBV) and Cytomegalovirus (CMV) were negative. A PET-CT scan showed splenomegaly and inflammatory lung lesions. Significant results for the patient’s initial blood work were listed in Table 1. Therefore, HLH was diagnosed based on both the HLH2004 diagnostic criteria (fulflling seven out of the eight criteria), and the patient undergone etoposide, methylprednisolone, and immunoglobulin treatment. Etoposide (weeks 1-2, 100 mg/ m2 /d twice a week; weeks 3-5, 100 mg/m2 /d once a week); methylprednisolone (days 1-3, 240 mg/d; days 4-7, 120 mg/d; week 2, 60 mg/d; weeks 3-5, 40 mg/d tapered to discontinuation). So far, the patient has been followed up for 4 months, the symptoms such as shortness of breath, fever completely disappeared, and the CBC and biochemical parameters returned to normal.
Table 1: Pertinent lab values on admission.
| Parameter | Results | Normal ranges | |
|---|---|---|---|
| Complete blood count | Day 0 | Day 30 | |
| White blood cell (109/L) | 1.88 | 3.92 | 3.69-9.16 |
| Neutrophil count (109/L) | 0.77 | 3.18 | 2.00-7.00 |
| Hemoglobin (g/L) | 90 | 75 | 113-151 |
| Platelet (109/L) | 28 | 156 | 100-300 |
| Coagulation | |||
| APTT (s) | 48.4 | 29.3 | 23.3-32.5 |
| PT (s) | 65.8 | 72 | 70.0-130.0 |
| Thrombin time (s) | 23.4 | 16.0 | 14.0-25.0 |
| Fibrinogen (g/L) | 1.25 | 2.66 | 1.80-3.50 |
| D-Dimer (mg/L) | 10.53 | 0.42 | <0.5 |
| Hepatic and renal function | |||
| ALT (U/L) | 111 | 11 | 0-40 |
| AST (U/L) | 501 | 13 | 0-45 |
| Total bilirubin (μmol/L) | 19.0 | 15.4 | 3.42-20.50 |
| Direct bilirubin (μmol/L) | 11.10 | 5.3 | 1.00-6.84 |
| Lactate dehydrogenase (U/L) | 3462 | 320 | 100-245 |
| Albumin (g/L) | 34.6 | 29.8 | 35-50 |
| Globulin (g/L) | 19.8 | 21.6 | 20.0-35.0 |
| Creatinine (μmol/L) | 62.0 | 21.0 | 35.0-97.0 |
| Fasting lipid | |||
| Triglycerides (mmol/L) | 5.62 | 1.2 | <1.7 |
| Total cholesterol (mmol/L) | 3.10 | 5.12 | 2.38-5.43 |
| HDL-C (mmol/L) | 0.22 | 0.92 | 1.00-2.20 |
| LDL-C (mmol/L) | 0.90 | 4.08 | 0.00-3.10 |
| Lymphocyte | subsets | ||
| NK cells (%) | 3.2 | Not detected | 3.33-30.47 |
| Infammatory factors | |||
| Ferritin (μg/L) | >40000.0 | 10.2 | 4.6-204.0 |
| hsCRP (mg/L) | 10.80 | 4.2 | 0-5 |
| sCD25 (U/ml) | 2869 | Not detected | 223-710 |
| IL-6 (pg/ml) | 2.7 | 2.2 | <5.30 |
| Virus | |||
| EB-VCA IgA | Negative | Not detected | Negative |
| EB-VCA IgM | Negative | Not detected | Negative |
| EB-VCA IgG | Negative | Not detected | Negative |
| Whole blood CMV DNA (copy/ml) | <400.00 | Not detected | <400.00 |
| SARS-CoV-2 RNA | Positive | Negative | Negative |
| Hepatitis B surface antigen | Negative | Negative | Negative |
| Hepatitis C virus antibody | Negative | Negative | Negative |
| HIV antibody | Negative | Negative | Negative |
| Anti-nuclear antibody spectrum | Negative | Negative | Negative |
| Color Doppler echocardiography | Negative imaging | Negative imaging | Negative imaging |
| HLH-2004 diagnostic criteria | 7 of the 8 criteria |
Cut-off: 5 of the 8 criteria |
Discussion
HLH is a clinical syndrome characterized by cytokine overproduction, resulting from excessive activation of macrophages and T cells due to various causes, and leading to an inflammatory cytokine storm [3]. HLH includes both primary HLH (pHLH) and secondary HLH (sHLH). pHLH is associated with genetic defects in the perforin and degranulation pathways while sHLH is caused by various factors, such as viral infections, malignant tumors, and autoimmune diseases [4]. Characteristically, hypercytokinemia leads to persistent fever, pancytopenia, coagulation disorders, organomegaly, and rapid progression to disseminated intravascular coagulation, multiorgan failure, acute respiratory distress syndrome, and subsequent death [5]. COVID-19 varies greatly in severity, with the main clinical features including fever, cough, muscle pain, and fatigue. Mild cases may be asymptomatic carriers, while severe cases can show respiratory distress and hypoxemia within a week of disease onset, with rapid progression to acute respiratory distress syndrome and multiple organ dysfunction [6]. The severity and mortality of COVID-19 are mainly attributed to high levels of inflammation and the subsequent cytokine storm, resembling HLH in clinical presentation. Thus, the COVID-19-induced cytokine storm and HLH share similar clinical features and laboratory findings [7]. When two diseases coexist, rapid disease progression makes it difficult to establish a clear diagnosis, which increases the mortality rate of patients. Therefore, early diagnosis is beneficial for the recovery of diseases.
Research showed that SARS-CoV-2 is one of the triggers of sHLH, and the incidence of sHLH is higher in severe COVID-19 patients [2]. The H-score assessment is used to distinguish suspected sHLH in COVID-19 patients. Meng et al. [8] proposed that thrombocytopenia (<101×109 /L), elevated ferritin (>1922.58 ng/ml), and triglyceride levels (>2.28 mmol/L) were identified as independent risk factors for sHLH in COVID-19 patients.
The frequently used treatment regimen for HLH is the HLH2004 protocol, which includes eight weeks of induction treatment with etoposide and corticosteroids. The main purpose of this induction therapy is stabilization of the overactive immune system and correction of the high cytokine levels. Corticosteroids are the preferred choice for infection-related HLH and usually used in combination with IVIG [9]. Notably, cytokine-targeted therapy is a potential treatment for HLH, as it can significantly suppress proinflammatory signal transduction, control inflammation, and prevention of further organ damage [10]. A study indicated that the IL-6 receptor inhibitor tocilizumab and the IL-1 receptor inhibitor anakinra were effective in the treatment of sHLH [11]. The COVID-19 Treatment Guidelines Panel recommends the use of dexamethasone, tocilizumab, and baricitinib as immunomodulators in hospitalized COVID-19 patients. Later randomized Controlled Trials (RCTs) showed improvement in clinical outcomes in severe COVID-19 patients using tocilizumab [12]. However, anakinra and GM-CSF inhibitors were not recommended to be used in patients with COVID-19 due to the lack of clinical evidence [13]. Furthermore, a retrospective study illustrated that anakinra and tocilizumab had been widely used for the treatment of COVID-19-associated HLH, and it was reported that tocilizumab was effective for the treatment of cytokine release syndromes, especially following Chimeric Antigen Receptor (CAR)-T cell therapy [14].
Our case illustrates that in patients with unexplained fever, remarkably with pancytopenia, coagulopathy, and splenomegaly, exclusion of HLH is crucial for the diagnosis. Diagnosis of HLH is difficult owing to atypical clinical symptoms in the early stages of the disease, and definitive diagnosis is even more challenging when combined with COVID-19. Consequently, we suggest that HLH should be suspected in COVID-19 patients with worsening clinical status and cytokines. These patients require immediate evaluation for HLH, as any delay may result in a serious deterioration of the patient’s condition. Timely treatment and intervention may significantly reduce the mortality rate.
Declaration of conflicting interest: The authors declare that there is no conflictof interest.
References
- Farquhar JW, Claireaux AE. Familial haemophagocytic reticulosis. Arch Dis Child. 1952; 27: 519-25.
- Wood H, Jones JR, Hui K, et al. Secondary HLH is uncommon in severe COVID-19. Br J Haematol. 2020; 190: e283-e285.
- Ponnatt TS, Lilley CM, Mirza KM. Hemophagocytic lymphohistiocytosis. Arch Pathol Lab Med. 2022; 146: 507-519.
- La Rosée P, Horne AC, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019; 133: 2465-2477.
- Kim YR, Kim DY. Current status of the diagnosis and treatment of hemophagocytic lymphohistiocytosis in adults. Blood Res. 2021; 56: 17-25.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395: 497-506.
- Soy M, Atagündüz P, Atagündüz I, et al. Hemophagocytic lymphohistiocytosis: A review inspired by the COVID-19 pandemic. Rheumatol Int. 2021; 41: 7-18.
- Meng M, Chen L, Zhang S, et al. Risk factors for secondary hemophagocytic lymphohistiocytosis in severe coronavirus disease 2019 adult patients. BMC Infect Dis. 2021; 21: 1-14.
- Morimoto A, Nakazawa Y, Ishii E. Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr Int. 2016; 58: 817-825.
- England JT, Abdulla A, Biggs CM, et al. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev. 2021; 45: 100707.
- Chu R, van Eeden C, Suresh S, et al. Do COVID-19 infections result in a different form of secondary hemophagocytic lymphohistiocytosis. Int J Mol Sci. 2021; 22: 2967.
- Remap-Cap Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021; 384: 1491-1502.
- Fadlallah MM, Salman SM, Fadlallah MM, et al. Hemophagocytic Syndrome and COVID-19: A Comprehensive Review. Cureus. 2023; 15: e36140.
- Kayaaslan BU, Asilturk D, Eser F, et al. A case of Hemophagocytic lymphohistiocytosis induced by COVID-19, and review of all cases reported in the literature [J]. J Infect Dev Ctries. 2021; 15(11): 1607-1614.