Research Article
Volume 3, Issue 6
Abnormal Changes of Cerebral Blood Flow in Hypertension with Different Risk Levels
Baijie Wang1#; Ge Zhang2#; Xiuchun Wang3 ; Chunyan Yu4 ; Qiang Li5 ; Weizhao Lu6 ; Feng Wang5*; Yan Shao5*
1Department of Medical Imaging, Cancer Hospital Chinese Academy of Medical Sciences Shenzhen Center, China.
2Department of Medical Imaging, Jinan Beicheng Hospital, China.
3Encephalopathy Department, Taiwan Traditional Chinese Medicine Hospital, China.
4Department of Medical Imaging, Longgang Central Hospital of Shenzhen, China.
5Department of Medical Imaging, The Second Affiliated Hospital of Shandong First Medical University, China.
6College of Radiology, Shandong First Medical University, China.
#These Authors Contributed Equally to this Work.
Corresponding Author :
Feng Wang & Yan Shao
Tel: +35-31368027;
Email: 168973832@qq.com & taishansy88@163.com
Received : Apr 30, 2024 Accepted : May 31, 2024 Published : Jun 07, 2024 Archived : www.meddiscoveries.org
Citation: Wang B, Zhang G, Wang X, Wang F, Shao Y, et al. Abnormal Changes of Cerebral Blood Flow in Hypertension with Different Risk Levels. Med Discoveries. 2024; 3(6): 1163.
Copyright: © 2024 Wang F & Shao Y. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective: The purpose of this study was to examine the different alterations in Cerebral Blood Flow (CBF) induced by hypertension across various risk levels.
Materials and methods: A total of 121 hypertensive patients were classified into low-risk (n=30), moderate-risk (n=30), high-risk (n=31) and extremely high-risk (n=30) categories according to cardiovascular risk factors and target organ damage. We had 32 healthy individuals serve as a control group. CBF values of the whole brain and specific regions were retrieved using an Arterial Spin-Labeled (ASL) sequence. The CBF of the control group and varying hypertensive groups were analyzed using a Dunnett-t test. Univariate linear regression analysis was utilized to evaluate the relationship between Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), and CBF across all subjects, with P-values corrected via the False Discovery Rate (PFDR).
Results: Compared to the control group, the low-risk and moderate-risk groups displayed decreased CBF in certain brain regions. Notably, the extremely high-risk group revealed a decrease in basal ganglia CBF (p=0.034), whereas the moderate-risk group presented an increase of CBF in several regions. No significant changes in CBF were noted in the high-risk group. Regression analysis found no significant correlation between DBP, SBP, and CBF across all groups (PFDR<0.05).
Conclusion: We demonstrates that effective and stable self-regulatory mechanisms of CBF compenstate for elevated blood pressure at severe stages of hypertension, but not in the initial stages marked by low and moderate risk factors.
Keywords: Hypertension; Cerebral Blood Flow (CBF); Arterial Spin Labeled (ASL); Systolic Blood Pressure (SBP); Diastolic Blood Pressure (DBP).
Introduction
The aging global population is contributing to a rise in the number of individuals diagnosed with hypertension [1]. Several factors are implicated in the development of hypertension, including hereditary factors, chronic mental stress, endocrine disruption, metabolic imbalances, age-related arterial hardening, and kidney-related causes [2]. Persistently high blood pressure can induce a range of pathological changes such as the hyperplasia and hypertrophy of the arterial wall’s Smooth Muscle Cells (SMC), lipid hyalinization, and amyloid accumulation. These alterations could result in the narrowing of the arterial lumen, vascular remodeling, diminution of small vascular networks, which culminating in brain autoregulation impairment and reduced Cerebral Blood Flow (CBF) [3-6]. Numerous studies have confirmed that hypertension generally reduces CBF in various regions including the whole brain, cortex, and hippocampus [7,8]. A study by Lidia et al. found a close resemblance between the CBF of pre-hypertensive and hypertensive patients, suggesting that CBF reduction in hypertensive patients occurs prior to observable changes in brain structure and morphology [9].
Despite previous research, there are still differing opinions on the relationship between blood pressure and CBF in patients with hypertension. Glodzik found a negative correlation between Systolic Blood Pressure (SBP) and CBF across the cerebral cortex and hippocampus in their entire study cohort, and a quadratic relationship between hippocampal CBF and SBP in hypertensive patients. Additionally, their findings indicated no significant correlation between CBF and Diastolic Blood Pressure (DBP) [10]. Shokouhi discovered a link between decreased CBF and cerebral cortex volume with increased DBP but had no association with SBP, and a reduction in white matter volume was related to both increased SBP and DBP [11].
While earlier research has established a connection between hypertension and reduced CBF, few have addressed how CBF changes across different stages of hypertension. Research has shown that the cerebral cortex and hippocampus of hypertensive patients respond variably to increasing blood pressure [10]. We hypothesize that these changes are not homogeneous across the brain’s functional regions during a blood pressure increase. The objective of this study is to examine how CBF changes across the entire brain and specific brain regions in patients across various stages of hypertension and delve into the quantitative variations of CBF. This study aims to enhance our understanding of how hypertension risk factors affect brain tissue and further investigate the relationship between DBP, SBP, and CBF to determine whether blood pressure influences CBF in hypertensive patients.
Material and methods
Subjects
This study received approval from the Shandong First Medical University Ethics Committee, and all subjects gave their informed consent.
We enrolled patients with primary hypertension treated at the Second Affiliated Hospital of Shandong First Medical University between December 2020 and August 2022. Patients with central nervous system diseases including Traumatic Brain Injury (TBI), intracranial tumors, infections, related surgical history, acute strokes, significant arterial stenosis (The lumen stenosis rate is over 70%) or malformation, psychiatric disorders, cognitive impairment were excluded. We also excluded individuals using psychotropic drugs, heavy alcoholics (defining heavy alcohol consumption as 100 g or more daily over at least three months), and those with incomplete MRI examinations. Ultimately, we included 121 patients, 59 males and 62 females, aged between 40 to 65. The duration of their hypertension ranged from one month to 20 years, and 70 patients had received or were currently on medication.
As a control group, we enlisted 32 healthy volunteers composed of 19 males and 13 females, aged from 46 to 65.
Blood pressure measurement and clinical data collection
Within 48 hours before or after their MRI examination, we collected patients’ Electrocardiogram (ECG), glucose, glycosylated hemoglobin, low-density lipoprotein, high-density lipoprotein, total cholesterol, homocysteine, and creatinine levels. Blood samples were collected under fasting conditions. SBP and DBP were measured ten minutes before MR examination using an Omron wrist electronic sphygmomanometer while the subjects were seated or lying. The patient was ensured to be in a calm, relaxing environment. The wrist strap was positioned one centimeter above the wrist line, ensuring moderate tension and alignment with the heart level. Measurements were taken three times and then averaged.
Image acquisition and processing
All brain MRI scans were conducted on a GE Discovery MR750 3.0T superconducting magnetic resonance with an 8-channel scanning coil. The scanning sequences included Magnetic Resonance Angiography (MRA), T1-Weighted Imaging (T1WI), T2-Weighted Imaging (T2WI), T2 Fluid-Attenuated Inversion Recovery (T2-FLAIR), Diffusion Weight Imaging (DWI), and Arterial Spin Labeling (ASL) sequences. MRA was conducted with the following parameters: Time of Repetition (TR) = 23 ms, Echo Time (TE) = 3.4 ms, field of view (FOV) = 220×194 mm2 , slice thickness = 1.4 mm, flip angle = 20° , vessel uniformity = 1.00. The scan parameters of the T1WI were: TR = 1750 ms, TE = 25.1 ms, FOV = 240×240 mm2 , slice thickness = 5 mm, acquisition matrix = 512×512. T2WI were acquired using: TR = 5810 ms, TE = 92 ms, FOV = 240×240 mm2 , slice thickness = 5 mm, acquisition matrix = 512×512. T2 FLAIR images were acquired with following parameters: TR = 8500 ms, TE = 145 ms, FOV = 240×240 mm2 , 20 contiguous slices with thickness of 5.0 mm, acquisition matrix of 256×256. The scan parameters of the DWI were: TR = 40.6 ms, TE = 50.4 ms, FOV = 240 mm × 240 mm2 , slice thickness = 2mm, acquisition matrix = 384×320. ASL parameters were as follows: sampling points on eight spirals, FOV = 220×220 mm2 ; reconstructed matrix = 128×128, TR = 4781 ms, TE = 11.1 ms, number of excitations = 3.0, slice thickness = 3.0 mm, labeling plane was positioned at the base of the cerebellum with labeling duration = 1,500 ms and post labeling delay = 1525 ms, 90 slices covering the whole brain.
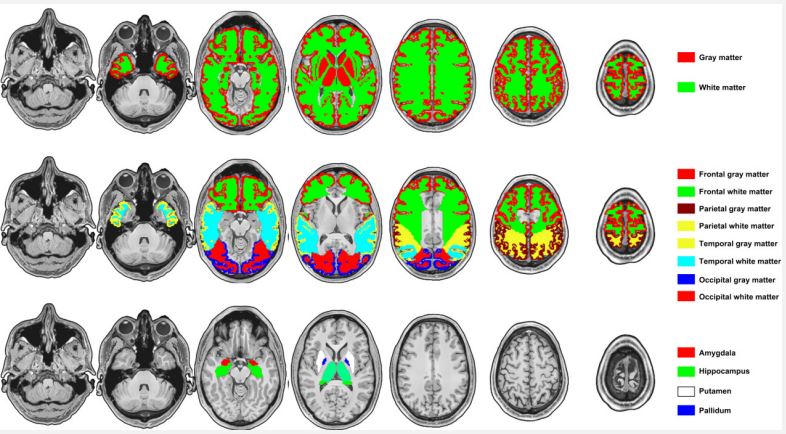
We calculated voxel-wise CBF maps from ASL data using Functool software (Version 9.4.05) on the MRI server. Primarily, the quantitative CBF values (ml/100 g/min) were evaluated from the control and label images based on the general kinetic model, considering the labeling duration, the longitudinal relaxation time of blood, and labeling efficiency [12]. The brain ROIs were defined using the WFU pickatlas tool embedded in the SPM12 software, which included: the whole brain, whole cerebral cortex, whole cerebral white matter, midbrain, pons, medulla oblongata, cerebellum, hippocampus, putamen, globus pallidus, lentiform nucleus, caudate nucleus, amygdala, claustrum, thalamus, corpus callosum, frontal cortex and white matter, temporal cortex and white matter, parietal cortex and white matter, and occipital cortex and white matter. In addition, the basal ganglia included the caudate nucleus, globus pallidus, putamen, substantia nigra, and subthalamic nucleus. The substantia nigra was defined using the mask provided by Depierreux et al. [13], and the caudate nucleus, globus pallidus, putamen, and subthalamic nucleus were defined using the WFU pickatlas tool. Finally, the basal ganglia mask was obtained by the union operation of the above region masks (Refer to Figure 1 for brain parcellation).
Cardiovascular risk stratification method
Based on the Chinese Guidelines for the Prevention and Treatment of Hypertension (2022), hypertension patients were segregated into low-risk, moderate-risk, high-risk, and extremely high-risk groups. The specific evaluation criteria are provided in Supplementary Tables S1 and S2.
Statistics analysis
We used SPSS 26.0 software for statistical analysis. We used SPSS 26.0 software for statistical analysis. Data were represented as mean ± standard deviation (M±SD) which obeyed a normal distribution and variance homogeneity. One-way ANOVA evaluated differences in age, SBP, and DBP among different hypertension subgroups and controls. Chi-square tests analyzed gender, hyperlipidemia, hyperglycemia, smoking, and drinking status differences among different hypertension subgroups and controls and Fisher’s exact probability method was used to correct. By applying the Dunnett-t test, we compared the CBF of the control group against various hypertension groups. Two independent samples T-test also be distinguished by gender within each group. Univariate linear regression analysis gauged the correlation between SBP, DBP, and CBF of whole brain and each brain region in all subjects, and FDR correction (PFDR<0.05) was applied to control false positives in the regression.
Results
General information
A total of 153 cases were analyzed, there are 32 healthy volunteers and incorporating 121 hypertensive patients categorized into low-risk (n=30), moderate-risk (n=30), high-risk (n=31), and extreme high-risk groups (n=30). Significant divergence existed in the proportion of hyperglycemia (χ²=4.71, P=0.021) and smoking (χ²=14.31, P=0.006) among the participants. Noticed differences in SBP (F=44.33, P=0.000) and DBP (F=36.17, P=0.000) emerged across different groups, while no statistical variation was found in age (F=2.38, P=0.054), sex (χ²=0.97, P=0.915), hyperlipidemia (χ²=1.65, P=0.800), or drinking (χ²=8.37, P=0.079) within various groups (Table 1).
Comparison of CBF in different brain regions between control group and different hypertension risk groups
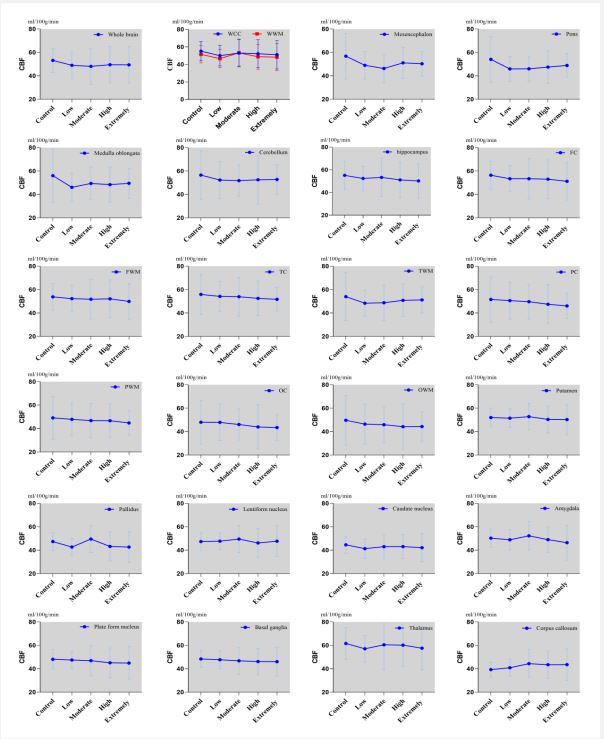
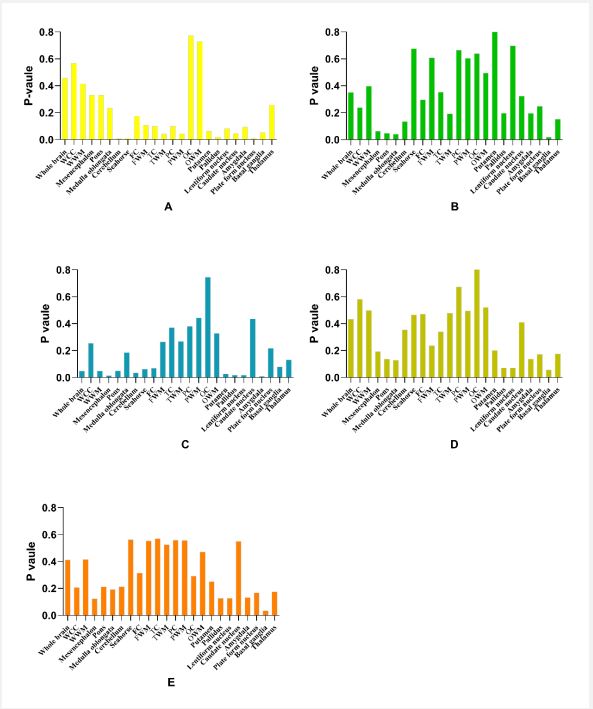
Regarding subgroups of hypertension, decreased CBF values were found in the medulla oblongata, pons, and basal ganglia of the low-risk group compared to the control group (P<0.05). The moderate-risk group registered lower CBF values in both the midbrain and cerebellum compared to the control group (P<0.05), but increased CBF in other areas including the entire brain, white matter, putamen, globus pallidus, lentiform nucleus, and amygdala. There was no notable difference in CBF across the whole brain and other regions between the high-risk group and control group (P>0.05). CBF value in the basal ganglia (p=0.034) was reduced in the extremely high-risk group relative to the control group (Figures 2 and 3).
Regression analysis between CBF of the whole brain and different brain regions, and DBP, SBP among the control group and different risk groups
No discernible associations were found among CBF of the whole brain and all brain regions with DBP or SBP in the participants (P>0.05) (Table S3, and S4).
Table 1: Demographic and clinical information.
| control group (n=32) |
low risk group (n=30) |
medium risk group (n=30) |
high risk group (n=31) |
extremely high risk group (n=30) |
χ² (F) | P value | |
|---|---|---|---|---|---|---|---|
| Age (M±SD) | 56.94±7.0 | 53.47±6.2 | 54.90±6.6 | 54.80±6.0 | 58.14±6.6 | 2.38 | 0.054 |
| Female/male | 13/19 | 16/14 | 15/15 | 15/16 | 16/14 | 0.97 | 0.915 |
| Hyperglycaemia (%) | 15.2 | 17.9 | 33.3 | 33.3 | 30.0 | 4.71 | 0.021 |
| Hyperlipidemia (%) | 24.2 | 20.5 | 33.3 | 29.2 | 20.0 | 1.65 | 0.800 |
| Smoking history (%) | 12.1 | 15.4 | 20.8 | 35.8 | 40.0 | 14.31 | 0.006 |
| History of Drinking (%) | 15.2 | 30.8 | 33.3 | 37.5 | 60.0 | 8.37 | 0.079 |
| SBP (M±SD) | 117.71±10.6 | 143.73±12.3 | 152.37±18.8 | 155.17±22.1 | 182.29±14.5 | 44.33 | 0.000 |
| DBP (M±SD) | 82.65±5.4 | 90.43±7.3 | 94.67±6.3 | 100.63±10.6 | 104.50±8.4 | 36.17 | 0.000 |
Note: P<0.05 indicates statistical significance.
WCC: Whole Cerebral Cortex; WWM: Whole White Matter; FC: Frontal Cortex; FWM: Frontal White Matter; TC: Temporal Cortex; TWM: Temporal White Matter; PC: Parietal Cortex; PWM: Parietal White Matter; OC: Occipital Cortex; OWM: Occipital White Matter
WCC: Whole cerebral cortex; WWM: whole white matter; FC: Frontal cortex; FWM: Frontal white matter; TC: Temporal cortex; TWM: Temporal white matter; PC: Parietal cortex; PWM: Parietal white matter; OC: Occipital cortex; OWM: Occipital white matter.
Discussion
Long-term hypertension can inflict damage upon the structure and function of the brain, triggering a range of illnesses that could eventually degrade quality of life or pose mortal threats. Such as Cerebral Small Vessel Disease (CSVD), Vascular Cognitive Impairment (VCI), Alzheimer’s Disease (AD), and cerebral infarction and so on. Vascular Cognitive Impairment (VCI) and Alzheimer’s Disease (AD) are prevalent conditions in the elderly population, with hypertension recognized as one of the common risk factor. Recent studies suggest that the age at which hypertension appears differentially impacts the onset of VCI and AD. Hypertension at mid-age has been found to trigger the onset of VCI and AD. However, hypertension in the elderly enhances Cerebral Blood Flow (CBF), thereby reducing the likelihood of VCI and AD development [5,14]. Multiple studies demonstrate that VCI and AD may be arise from the decrease in CBF due to hypertension [15-18], but some studies propose a stronger correlation between these diseases’ and a reduction in Cerebrovascular Reactivity (CVR) than with decrease in CBF [19].
The brain is an intricate structure with many diverse functional regions. Damage to these areas can lead to corresponding dysfunctions, such as decreased CBF in the frontal lobe, parietal lobe, middle temporal lobe, and putamen, potentially resulting in cognitive impairment [20]. Research suggests that hypertensive patients might experience a decline in CBF, but these decreases do not uniformly affect all brain regions [7,8]. Varying responses to changes in blood pressure are noted across different brain regions [10], and hypertension predominantly leads to a decrease in CBF in the anterior circulation supply area in the early stages [21,22]. Therefore, we hypothesize that the response of CBF across different functional regions of the brain to hypertension varies. To prove this hypothesis, we conducted regional observations of the brain in our study to comprehend how different functional areas respond to increased blood pressure.
Through our research, we found that Cerebral Blood Flow (CBF) had decreased in numerous brain regions among subgroups at risk of hypertension, compared to the control group. The decrease in CBF in prolonged hypertension cases is mainly due to abnormalities in the arterial wall structure, coupled with a diminishing self-regulation capacity [3]. Abnormalities in the arterial wall are triggered mainly by three mechanisms: firstly, enduring hypertension can prompt and hasten arterial wall atherosclerosis, leading to fibrin-like necrosis in penetrating arteries and arterioles, thus causing stenosis or blockage which in turn results in reduced distal blood flow [23,24]. Secondly, hypertension can cause negative remodeling of arterioles, increasing vascular resistance while maintaining normal CBF levels. When blood vessel pressure increases initially, it prompts local CBF reduction as hypertension persists - particularly in areas like the temporo-occipital lobe, prefrontal cortex, and hippocampus [25,26]. Lastly, hypertension causes capillaries to become less dense, meaning they become sparse and thin - this results in a corresponding decrease in CBF values [6].
The primary reasons for impaired cerebral vascular self-regulation are abnormal vascular structures such as arteriosclerosis and wall remodeling prompted by hypertension, which reducing myogenic regulatory capabilities in cerebral . Hypertension also leads to abnormal local metabolic or neurogenic factors, hampering the CBF self-regulation [23,24]. For instance, hypertension can reduce local amounts of Nitric Oxide (NO), prostacyclin, endothelium-derived factors, etc. in cerebral, leading to vascular endothelial dysfunction and a drop in CBF [27].
Our findings illustrate that CBF anomalies, seen in hypertensive patients, primarily occur within groups at low and moderate risk after categorizing patients into different risk levels. The vascular Smooth Muscle Cells (SMCs) of small brain arteries can detect increases in Cerebral Perfusion Pressure (CPP) and contract to narrow the lumen, thereby maintaining the stable of CBF in hypertensive patients when blood pressure rises. The mechanism of this self-regulation also involves changes in the arteriole endothelium, ions, autonomic nervous system, and hormonal system [5,23,28-30]. We hypothesize that the impact of CBF self-regulation in low-risk and medium-risk groups is inconsistent, and the amount of CBF self-regulation varies across different brain regions, leading to inconsistent changes in CBF throughout each region. In high-risk and extremely high-risk groups, however, the effect of CBF self-regulation appears to become more stable and effective. This is likely because, during severe stages of hypertension, the body’s self-regulating mechanisms play a critical protective role in maintaining CBF stability. In the extremely high-risk group, we observed a drop in CBF only within the basal ganglia. It remains to be determined, through future research, whether this decrease will manifest in other brain regions over time as hypertension persists, and whether it would occur upon the onset of a stroke.
Lassen et al. conducted a study that resulted in a CBF-pressure curve, known as the Lassen curve. This curve indicated that when arterial pressure was within a range of approximately 100±50 mmHg, CBF remained fairly stable and exhibited a plateau-like trend. Later, this phenomenon was referred to as CBF automatic regulation. This form of regulation proved more effective when the average arterial pressure slowly fluctuated around 90±20 mmHg [31-33]. Strandgaard et al. discovered that persistent hypertension could cause a rightward shift in the Lassen curve, and the lower limit of automatic regulation would correspondingly rise. This led to a linear relationship between blood pressure and CBF in the lower blood pressure what was originally in the left of the Lassen curve’s plateau region [34]. We observed fluctuations in the low and moderate-risk groups, but not in the high-risk and extremely high-risk groups. A possible explanation is that the self-regulation of CBF has established an effective and stable compensatory mechanism in response to blood pressure increases during severe hypertension stages.
Based on the literature, Cerebral Blood Flow (CBF) in hypertensive patients is lower compared to healthy individuals [7,8,20]. In our study, even though there’s a noted reduction in CBF within the basal ganglia of the extremely high-risk group, but no significant statistical difference was found between the high-risk group and the control group, and this observation doesn’t align perfectly with the existing literature. We suspect that the cause of these findings might stem from our observation group’s exclusion criteria specifically left out patients with strokes and significant arterial stenosis, to avoid the self-regulation effect of CBF is masked by the stroke and severe vascular stenosis. We plan to include these patients in future research endeavors to more closely monitor the trajectory of CBF changes across various stages in hypertensive.
Research has established a clear linear relationship between blood pressure and blood flow in the heart and major blood vessels [35,36], however, the correlation between blood pressure and CBF is different from that and more complicated. To maintain regular brain function, a consistent supply of blood flow is necessary. In healthy individuals, the curve of the relationship between blood pressure and CBF features a plateau period, thus ensuring stable CBF even when blood pressure fluctuates within a certain range [5,23,33]. This stability of CBF is primarily achieved through CBF self-regulation, triggered by the varying responses of the brain’s small arteries to changes in blood pressure and other factors [5]. For instance, neurotransmitter receptor expression varies in the same vascular tree, causing disparate contractions or expansions from the same neurotransmitter in larger arteries (like the middle cerebral artery) and smaller brain arteries.
Previous studies have posited a potential association between CBF and either Systolic Blood Pressure (SBP) or Diastolic Blood Pressure (DBP), but conclusions are not uniform [10,11,37]. Our research did not find any correlation between CBF and SBP or DBP across risk groups. Studies showed that the Lassen curve shifted to the right in the hypertensive patients [31-34]. We conjecture that blood pressure correlates with CBF only when the plateau of Lassen curve shifts to the right beyond the area where we observed blood pressure. Our findings indicate that CBF alterations in hypertensive patients are inconsequential from the onset of hypertension, making it challenging to gauge the degree of impaired CBF self-regulation when selecting hypertensive observation subjects. Research results may differ or even contradictory due to inconsistent degree of CBF damage within the observation group. On the other hand, several studies suggest that certain medications can effectively enhance the CBF in hypertensive patients [37,38]. Therefore, different results might be due to the potential impact of these drugs, which are difficult to rule out when selecting hypertensive patients given the prevalence of early hypertension diagnosis and treatment. Additionally, our study did not include hypertensive patients with stroke and significant arterial stenosis, leading to different outcomes due to some patients with severe impairment of CBF self-regulation were excluded, Moreover, the relatively narrow blood pressure range of the patients we observed may be one of the reasons for this result. Further research is necessary to validate this supposition.
There were some limitations to our study that need addressing. Each group contained a relatively small number of cases after dividing the participants based on risk level. Which may have influenced the results such as it weren’t real-time blood pressure measurements and clinical medication wasn’t included in the observational items. Severe hypertension complications such as stroke weren’t included in our research, and the blood pressure range of the patients we observed was narrow, and the duration of hypertension history wasn’t accounted for as an observation item, as the onset time of hypertension often doesn’t align with the time of hypertension diagnosis. Research in these areas needs to be addressed in the future.
Conclusion
Our study demonstrates that CBF abnormalities manifest in hypertensive patients is diferent across various risk categories and there were fluctuations in the low and moderate-risk groups, but not in the high-risk and extremely high-risk groups. Moreover, our findings suggest that DBP and SBP may not be the primary risk factors influencing CBF. Future studies will delve deeper into the self-regulatory properties of CBF poststroke and the impact of other factors, such as the duration of hypertension, on CBF.
References
- BC Rossier, M Bochud, O Devuyst. The hypertension pandemic: An evolutionary perspective, Physiology. 2017; 32(2): 112-125. doi: 10.1152/physiol.00026.2016.
- Y Huang, et al. Association of all-cause and cardiovascular mortality with prehypertension: A meta-analysis American Heart Journal. 2014; 167(2): 160-168. doi: 10.1016/j.ahj.2013.10.023.
- S Wu, et al. Cardiovascular events in a prehypertensive Chinese population: Four-year follow-up study, International Journal of Cardiology. 2013; 167(5): 2196-2199. doi: 10.1016/j.ijcard.2012.05.123.
- K W Nam, H M Kwon, H Y Jeong, et al. Cerebral Small Vessel Disease and Stage 1 Hypertension Defined by the 2017 American College of Cardiology/American Heart Association Guidelines, Hypertension (Dallas, Tex: 1979). 2019; 6: 1210-1216. doi: 10.1161/HYPERTENSIONAHA.119.12830.
- N Yazdani, MS Kindy, S Taheri. CBF regulation in hypertension and Alzheimer’s disease. Clinical and Experimental Hypertension. 2020; 42(7): 622-639. doi: 10.1080/10641963.2020.1764014.
- N Elsaid, et al. Impact of stress and hypertension on the cerebrovasculature, Frontiers in Bioscience – Landmark. 2021; 26(12): 1643-1652. doi: 10.52586/5057.
- S Dolui, et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral Blood Flow Secondary Analysis of the SPRINT MIND Randomized Clinical Trial, JAMA Neurology. 2022; 79(4): 380-389. doi: 10.1001/jamaneurol.2022.0074.
- IN Christie, et al. Cerebral perfusion in untreated, controlled, and uncontrolled hypertension, Journal of Cerebral Blood Flow and Metabolism. 2022; 42(12): 2188-2190. doi: 10.1177/0271678X221124644.
- L Glodzik, et al. Effects of vascular risk factors, statins, and antihypertensive drugs on PiB deposition in cognitively normal subjects, Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring. 2016; 2: 95-104. doi: 10.1016/j.dadm.2016.02.007.
- L Glodzik, et al. Different Relationship between Systolic Blood Pressure and Cerebral Perfusion in Subjects with and without Hypertension, Hypertension. 2019; 73(1): 197-205. doi: 10.1161/HYPERTENSIONAHA.118.11233.
- P Mahsa Shokouhi, P Deqiang Qiu, M Ayman Samman Tahhan, et al. Differential Associations of Diastolic and Systolic Pressures with Cerebral Measures in Older Individuals with Mild Cognitive Impairment Running, American Journal of Hypertension. 2018; 31(12): 1268-1277. doi: 10.1093/ajh/hpy104/5059691.
- Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015; 73(1): 102-116.
- Corrada MM, Hayden KM, Paganini-Hill A, et al. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study.Alzheimers Dement. 2017; 13(2): 103-110. doi: 10.1016/j.jalz.2016.09.007.
- CM Holland, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging, Stroke. 2008; 39(4): 1127-1133. doi: 10.1161/STROKEAHA.107.497438.
- V H Ten Dam, et al. Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities, Radiology. 2007; 243(1): 198-203. doi: 10.1148/radiol.2431052111.
- H Liu, et al. Changes in brain lateralization in patients with mild cognitive impairment and Alzheimer’s disease: A resting-state functional magnetic resonance study from Alzheimer’s disease neuroimaging initiative, Frontiers in Neurology. 2018; 9. doi: 10.3389/fneur.2018.00003.
- MC Eldaief, et al. Atrophy in bvFTD spans multiple large-scale networks in prefrontal and temporal cortex, Alzheimer’s & Dementia. 2021; 17(S4): 1-2. doi: 10.1002/alz.055338.
- Kim D, Hughes TM, Lipford ME, et al. Relationship between Cerebrovascular Reactivity and Cognition Among People With Risk of Cognitive Decline. Front Physiol. 2021; 12: 645342. doi: 10.3389/fphys.2021.645342.
- Zhang D, Zhang J, Zhang B, et al. Association of Blood Pressure, White Matter Lesions, and Regional Cerebral Blood Flow , Med Sci Monit. 2021; 27: e929958. doi: 10.12659/MSM.929958.
- Jefferson AL, Liu D, Gupta DK, et al. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology. 2017; 89(23): 2327-2334. doi: 10.1212/WNL.0000000000004707.
- Mahsa Shokouhi, Collin Clarke, Patricia Morley-Forster, et al. Structural and Functional Brain Changes at Early and Late Stages of Complex Regional Pain Syndrome. J Pain. 2018; 19(2): 146-157. doi: 10.1016/j.jpain.2017.09.007.
- KA Walker, MC Power, RF Gottesman. Defining the Relationship between Hypertension, Cognitive Decline, and Dementia: A Review, Current Hypertension Reports. 2017; 19(3). doi: 10.1007/s11906-017-0724-3.
- K Hayashi, T Naiki. Adaptation and remodeling of vascular wall; biomechanical response to hypertension, Journal of the Mechanical Behavior of Biomedical Materials. 2009; 2(1): 3-19. doi: 10.1016/j.jmbbm.2008.05.002.
- Y Huang, et al. Prehypertension and incidence of cardiovascular disease: A meta-analysis, BMC Medicine. 2013; 11(1): 1-9. doi: 10.1186/1741-7015-11-177.
- B V Zlokovic. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders, Nature Reviews Neuroscience. 2011; 12(12): 723-738. doi: 10.1038/nrn3114.
- EC Peterson, Z Wang, G Britz. Regulation of cerebral blood flow, International Journal of Vascular Medicine. 2011. doi: 10.1155/2011/823525.
- JAHR Claassen, DH J Thijssen, RB Panerai, FM Faraci. Regulation of cerebral blood flowin humans: Physiology and clinical implications of autoregulation, Physiological Reviews. 2021; 101(4): 1487-1559. doi: 10.1152/physrev.00022.2020.
- CK Willie, YC Tzeng, JA Fisher, PN Ainslie. Integrative regulation of human brain blood flow. Journal of Physiology. 2014; 592(5): 841-859, 2014, doi: 10.1113/jphysiol.2013.268953.
- L J Jensen, N H Holstein-Rathlou. The vascular conducted response in cerebral blood flow regulation, Journal of Cerebral Blood Flow and Metabolism. 2013; 33(5): 649-656. doi: 10.1038/jcbfm.2013.25.
- ML Lassen, et al. Assessment of attenuation correction for myocardial PET imaging using combined PET/MRI, Journal of Nuclear Cardiology. 2019; 26(4): 1107-1118. doi: 10.1007/s12350-017-1118-2.
- MC Tolcher, KA Fox, H Sangi-Haghpeykar, et al. Intravenous labetalol versus oral nifedipine for acute hypertension in pregnancy: effects on cerebral perfusion pressure, American Journal of Obstetrics and Gynecology. 2020; 223(3): 441.e1-441.e8. doi: 10.1016/j.ajog.2020.06.018.
- A Akhondi-Asl, FW Vonberg, CC Au, RC Tasker. Meaning of intracranial pressure-to-blood pressure Fisher-transformed Pearson correlation-derived optimal cerebral perfusion pressure: Testing empiric utility in a mechanistic model,” Critical Care Medicine, vol. 46, no. 12, pp. E1160-E1166, 2018, doi: 10.1097/CCM.0000000000003434.
- S Strandgaard, J Olesen, E SkinhøjJ, NA Lassen. Autoregulation of Brain Circulation in Severe Arterial Hypertension, British Medical Journal. 1973; 1(5852): 507-510, 1973, doi: 10.1136/bmj.1.5852.507.
- Jonathan Stone, Daniel M Johnstone, John Mitrofanis, Michael O’Rourke. The mechanical cause of age-related dementia (Alzheimer’s disease): The brain is destroyed by the pulse. J Alzheimers Dis. 2015; 44(2): 355-73. doi: 10.3233/JAD-141884.
- Safar ME, Boudier HS. Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension. 2005; 46(1): 205-9. doi: 10.1161/01.HYP.0000167992.80876.26.
- Van Dalen JW, Mutsaerts HJ, Petr J, et al. Longitudinal relation between blood pressure, antihypertensive use and cerebral blood flow, using arterial spin labelling MRI.J Cereb Blood Flow Metab. 2021; 41(7): 1756-1766. doi: 10.1177/0271678X20966975.
- Dolui S, Detre JA, Gaussoin SA, et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral Blood Flow: Secondary Analysis of the SPRINT MIND Randomized Clinical Trial. JAMA Neurol. 2022;79(4): 380-389. doi: 10.1001/jamaneurol.2022.0074.