Research Article
Volume 3, Issue 5
Investigation of Clock Genes Expression in G-CSFMobilized Individuals at Different Hours of the Day
Sanaz Aghajani1 ; Alireza Farsinejad1 ; Elham Roshandel2 ; Abbas Hajifathali2*
1Department of Hematology, Faculty Allied Health, Kerman University of Medical Sciences, Kerman, Iran.
2Hematopoietic Stem Cell Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Corresponding Author :
Abbas Hajifathali
Tel: +98-2123031657 & +98-2122432570;
Email: abbas.hajifathali33@gmail.com
Received : Apr 27, 2024 Accepted : May 21, 2024 Published : May 28, 2024 Archived : www.meddiscoveries.org
Citation: Aghajani S, Farsinejad A, Roshandel E, Hajifathali A. Investigation of Clock Genes Expression in G-CSF-Mobilized Individuals at Different Hours of the Day. Med Discoveries. 2024; 3(5): 1159.
Copyright: © 2024 Hajifathali A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective: Studies have shown that the mobilization of Hematopoietic Stem Cells (HSCs) is continuous and dependent on the circadian rhythms. In this study we have aimed to investigate of clock genes including PER-1, PER-2, PER-3 and CRY-1 expression in G-CSF-mobilized individuals at different hours of the day.
Methods: In this descriptive study, 15 healthy bone marrow transplantation donor referring to the Taleghani bone marrow transplantation center, Tehran, Iran between Jan 2019 and Feb 2020 were selected. The Leukocyte counts, CD34+ cell counts, PER1, PER2, PER-3, and CRY-1 expression were investigated at the morning and night of Day 0 (before G-CSF injection) and Day 4 after G-CSF injection.
Results: The expression of PER1 and PER-2 did not show significant differences in the morning and night before and after GCSF injection, but PER-3 and CRY-1 before and after injection more expressed in the morning than in the night. Besides, There were not any significant correlation between the PER1 or PER2 expression and CD34+ cell counts, but the higher expression of the PER-3 and CRY-1 in the morning than in the night is directly correlated with increasing the number of Leukocytes and CD34+ cells in the morning compared to the night.
Conclusion: Our findings indicated that the clock genes including PER1 and PER2 have not any significant roles in mobilization of HSCs from bone marrow to peripheral blood, but PER-3 and CRY-1 have a direct impact on mobilization of Leukocytes and HSCs.
Keywords: PER1; PER2; PER-3; CRY-1; Circadian rhythms; Hematopoietic stem cells; Mobilization.
Introduction
Normal Hematopoietic Stem Cells (HSCs) located in a special micro-environment called niche that regulates cell survival and function. There are two distinct niches in the bone marrow that include the vascular and endosteal niches. The osteoblastic niche located on the inner surface of the bone marrow, and filled with osteoblasts. The vascular niche, which is adjacent to the osteoblastic niche, consists of sinusoidal endothelial cells lining blood vessels and induces proliferation and differentiation in short-term HSCs [1]. Typically, most Hematopoietic Stem Cells (HSCs) are located in the bone marrow, but HSCs can also circulate at low frequencies in the peripheral blood. Early studies showed that Hematopoietic Stem Cells (HSCs) increased in the blood circulation in the patients recovering from chemotherapy, which led to the understanding that HSCs and HPCs could induced into the bloodstream, phenomenon called Mobilization. These mobilized cells can be collected and injected back into the bone marrow. At present, mobilized HSCs is the main source of hematopoietic cells transplantation [2].
Studies have shown that the movement of HSC is continuous and dependent on the circadian rhythms, so that the brightness or darkness affects the entry of HSCs into the peripheral blood or homing in the bone marrow [3]. Circadian rhythms regulated by the nervous system [3]. Hence, catecholamines play an important role in the excretion of HSCs from bone marrow, so that the removal of nerve endings in the bone marrow bed resulted in a sharp decrease in HSC mobilization, which under these conditions G-CSF injection does not have an effect on increasing the peripheral HSCs [4-8]. Neural terminals penetrate into the bone marrow along with vascular arteries and making a close contact with endosteal and vascular niches [9]. Living organisms, including humans, have an internal biological clock that helps them adapting to normal circadian rhythms. Human circadian rhythms in an internal rhythms with a period of about 24 hours, driven by environmental signs such as light / dark circles. The main center of the circadian rhythms coordination located at the Suprachiasmatic Nucleus (SCN) in the hypothalamus. The circadian rhythms regulate many biological activities of humans. Recent advanced studies have dramatically demonstrated the role of genetic factors in the SCN activities [10].
In SNC, clock genes like PER-1, PER-2, PER-3, CRY-1, CRY-2, ARNTL / BMAL1 and CLOCK are participated in transcription and translation feedback loops. In particular, CLOCK and BMAL1, the main transcription factor of the circadian rhythm activates, inducing the expression of PER-1, PER-2, PER-3, CRY1, CRY-2 through the attachment to the E-box motifs in the Enhancer region [11-13]. In the present study we have aimed to investigate of PER-1, PER-2, PER-3, and CRY-1 genes expression in G-CSF-mobilized individuals at different hours of the day.
Material and methods
Study design
In this descriptive study, 15 healthy bone marrow transplantation donor referring to the Taleghani bone marrow transplantation center, Tehran, Iran between Jan 2019 and Feb 2020 were selected. All participants in the study have not any sleep disorders, mental illness, and depression, and did not consume any sedative, hypnotic, or medications that affect the nervous system. The subjects have natural patterns of sleep. Healthy donor at 9 a.m and 9 p.m before starting the G-CSF drug (day zero) were considered as the control group. All of the participants signed the informed consent.
Measurements
Flow cytometry: To determine the purity of isolated CD34+ cells, flowcytometry was used. To do that, About 50 μl blood sample containing EDTA was transferred to an 1.5 mL tube and 25 μl reagent 1 (Anti Human CD45/FITC + Anti Human CD34/ RPE) was added to the tube. After adding, they were incubated for 30 minutes at 2-8°C. After the end of incubation time, 1 ml reagent 2 (Easy lyse diluted) was added and mixed quickly, and Incubated in the dark and at room temperature, then 50 μl reagent 3 (Cyto CountTM ) was added and immediately read by flowcytometry. For control sample (Isotype Control), instead of the CD34-PE antibody, a negative control solution (Mouse IgG1) was used. It should be noted that besides the control of isotype, untreated control was also used.
RNA extraction: In order to extract RNA, first, one milliliter of thiazole solution was added onto the cells, well mixed and incubated for 5 minutes at room temperature. 200 μl of chloroform was added to the above mixture after transfer to micro tubes, and with hand shaking well blended for 15 minutes. The mixture was incubated for 4 minutes at room temperature and then centrifuged for 20 minutes at 4°C and 13200 rpm. The supernatant of the 3-phase product was removed, transferred to a new micro tube and added to 500 μl isopropanol, the mixture was incubated 10 min at room temperature. It was then centrifuged at 4°C for 10 minutes at 12000 rpm. The supernatant was discarded and white precipitate was mixed with 1 ml of cold ethanol 75%. The mixture was centrifuged at 4°C for 5 minutes at 7500 rpm. Ethanol was deposited on a precipitate and placed at room temperature to dry as much as possible. The precipitate was dissolved in 20 μl of sterile water. Finally, the concentration of extracted RNA was determined.
qRT PCR: In this study, we used a two-step SYBR Green method, which first produced a cDNA genomic RNA and then converted cDNA into two-stranded DNA in a separate tube. CyberGreen’s detecting system is the simplest, most commonly used and most cost-effective method, based on the ability of cyber-green to split the double-stranded DNA check and subsequently increase fluorescence up to 2000 times the initial free value. In this study, GAPDH gene was selected as the Housekeeping gene. The sequence of primers used in the study was summarized in Table 1. In this study, the GreenPCR Master Mix Kit (ABI) was used. The reaction was prepared using 0.75 μl of each of the primers, 10 μL SYBR Green, 13.5 μL ddW, 2 μL cDNA at in the special tubes. The PCR was performed according to the specific temperature pattern, 1 minute at 95°C, 30 seconds at 58°C, 15 seconds at 72°C, plate readings, and 40 repetitions of from the Step 2 in Step-one ABI instrument.
Statistical analysis
Gene expression profile was calculated by 2ΔΔCt- method. Data were analyzed by SPSS 23 software. The mean and standard error of the mean were calculated. Mean values were compared using Paired Samples t-test. The data were reported as mean ± SD. P value less than 0.05 considered significant.
Results
In this study, 15 healthy donors (8 males and 7 females) with an average age of 38 years were studied. Characteristics of patients’ circadian rhythms, the count of total WBC and CD34+ cells are summarized in Table 2.
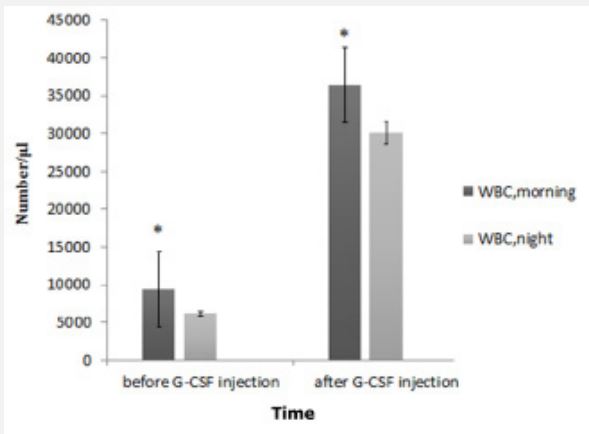
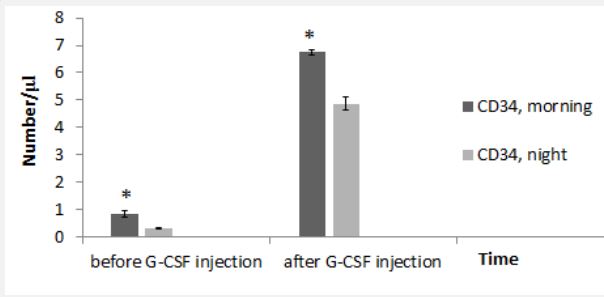
Before the G-CSF injection, morning WBC count had significant difference with WBC at night. Similarly, after the GCSF injection, morning WBC significantly were higher than WBC at night (Figure 1). Before the injection of GCSF, there was significant difference in the count of CD34+ cells in the morning compared to that at night. Also, after GCSF injection, there was significant difference in the count of CD34+ cells in the morning compared to that at night (Figure 2).
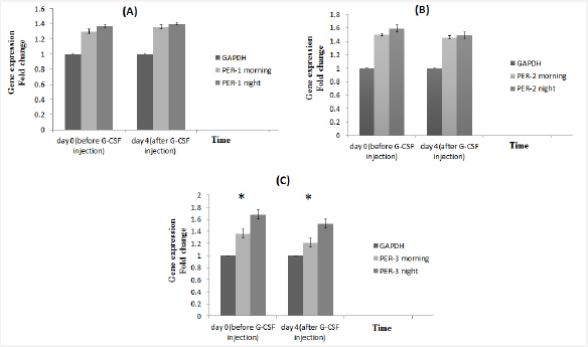
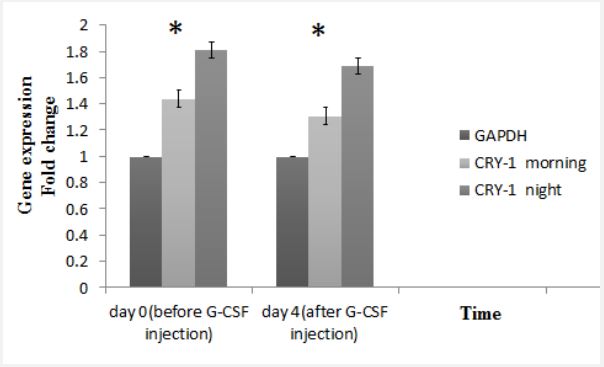
There was no significant difference in expression of PER-1 in the morning and night before and after GCSF injection (Figure 3A). Also, PER-2 expression did not show any significant changes during the study (Figure 3B). But in the control and target groups, CRY-1 expression was higher in the morning before and after the G-CSF injection. And the drug had no significant effect on gene expression (Figure 3C). As mentioned, the number of CD34+ cells was higher in the morning than in the night. Considering the effect of circadian rhythms on the expression of clock genes and its effect on mobilization, it can be stated that the increase in the amount of stem cells in the morning may be related to the increase in CRY-1 expression. According to the evaluation, PER-3 expression level, same as CRY-1 expression, is higher in the morning than in the night (Figure 4).
Discussion
Different drugs and agents are used to mobilize HSCs s from bone marrow to peripheral blood. The G-CSF has the ability to move bone marrow cells to the peripheral blood [14]. However, mechanisms that increase hematopoietic stem cell elevation following G-CSF injection and the role of circadian rhythms on the G-CSF effects are still unknown. Published studies suggested various factors such as the effect of the expression of clock genes, changes in circadian rhythms, and bone marrow niche alteration that affects G-CSF efficacy. The bone marrow microenvironment consists of cellular elements and extracellular matrix. The loss of attachment of hematopoietic cells to cellular elements in the BM niche plays a major role in the mobilization and migration of these cells to the peripheral blood [15]. The exact functioning of stem cell migration inducing drugs has not yet been fully elucidated. The stimulation of the bone-marrow cells may be the response to the signals from the circadian rhythms and the effects of the sympathetic nervous system. Several studies have been carried out on the role of the nervous system in the mobilization of hematopoietic stem cells to peripheral blood. These data suggest that signals from the nervous system are essential for the movement of stem cells from bone marrow to peripheral blood, and in mice whose nervous system has deleted, stem cell mobilization does not occur. This study rejected the role of proteolytic enzymes as the main factor in G-CSF action, and have shown that in cases of defective enzymes, the mobilization of these cells occurs [16]. During the mobilization, G-CSF interacts with at least two target cells:
Cells that induce the production of proteases and affect the integrity of the BM extracellular matrix, and Sympathetic neurons that nerves to osteoblastic cells and other stromal cells, including mesenchymal cells and the endothelial cells. These neurons are stimulated through unknown mechanisms and secreted noradrenaline that affected the function and morphology of osteoblasts. Subsequently the production of CXC12 is reduced, the release of stem cells to the peripheral blood increase [17].
Table 1: Primer sequences.
| Primer | Primer sequences |
|---|---|
| CRY-1 Forward | ACGATATAGAGGACTAGGTCTTCTGGC |
| CRY-1 Reverse | CCCTTGAGAGCAACTTCCACTG |
| PER-1 Forward | ATTTAGGTTTACGTGCGTTC |
| PER-1 Reverse | CGACTCAAAAACGAAAATCG |
| PER-2 Forward | GCGGTTTCGTTGCGGTTTAC |
| PER-2 Reverse | GCCGACGCCGTTTCAAACCG |
| PER-3 Forward | CGACAGTGTCATCAGATACCTGAAG |
| PER-3 Reverse | GATTTGCAAACCAGCTTGTAAGG |
| GAPDH Forward | CCTGGCGTCGTCATTAGTAGTG |
| GAPDH Reverse | TCAGTCCTGTCCATAATTAGTCC |
Table 2: Donor profile.
| Average | Range | Variable |
|---|---|---|
| 38 | 51-25 | Age |
| 8 males / 7 females | Sex | |
| 23:00 | 1:00-22:00 | Sleeping time |
| 07:15 | 9:00-6:30 | Waking hours |
| 13:00 | 14:00-12:00 | Eating lunch hours |
| 20:30 | 21:00-20:00 | Eating dinner hours |
| 9439 | 5300-10300 | WBC total count (day 0, morning) |
| 6174 | 4720-9150 | WBC total count (day 0, night) |
| 36487 | 26300-48100 | WBC total count (day 4, morning) |
| 30060 | 21100-45200 | WBC total count (day 4, night) |
| 0.84 | 0.49-1.2 | CD34+ count (day 0, morning) |
| 0.32 | 0.16-0.7 | CD34+ count (day 0, night) |
| 6.75 | 4.47-9.2 | CD34+ count (day 4, morning) |
| 4.87 | 3.79-7.43 | CD34+ count (day 4, night) |
For the first time in 1983, Ashkov stated that circadian rhythms, especially light and darkness had a direct effect on all physiological processes of animals and humans [18]. Environmental effects are applied by regulating the internal biological molecular and clocks genes, a highly protected mechanism during the evolution of organisms. In mammals, the clock genes are located in a collection of 20,000 neurons in the anterior part of the hypothalamus. Kjuchen et al. (2003) stated that the signals received by light receptors in the retina affect the expression of genes in other tissues by regulating the expressions of different neurotransmitters such as serotonin, adrenaline and noradrenaline [19]. Moreover, Brown and Vaughan showed that the clock genes expressed in all body tissues, and the 24-hour circadian rhythms regulate the physiology of the tissues [20].
Matsu et al. (2003) stated that clock genes play a key role in cell cycle and cell division. Finally, Schirmerman et al. reported that the changes in expression of the clock genes under the influence of 24-hour clock rhythms played a major role in the differentiation and migration of stem cells [21,22]. MendezFerrer et al. stated that the flow of stem cells from bone marrow to peripheral blood (mobilization) and vice versa, are affected by circadian rhythms. In humans, the release of hematopoietic stem cells reaches to the maximum level in the early hours of the day. This rhythm is regulated by changes in central clock genes, adrenaline and noradrenaline secretion from the sympathetic nerves in the bone marrow niche. It has been reported that the aging and some diseases can affect these fluctuations in the cellular flow and reduce the amount of mobilization [5,23].
In the present study, at first, to examine the effects of circadian rhythms on blood cell mobilization, blood counting by CBC assay and evaluation of the number of CD34+ stem cells at two different times in the morning and night before G-CSF injection and on the fourth day after G-CSF drug injection was analyzed. The results showed that the number of circulating cells in the morning shown significant differences compared with the cell counts at night. Similarly, in post-injection samples, the number of circulating cells in the morning was clearly higher than the night. The circadian rhythms has no effect on the circulating cell counts before and after the G-CSF injection.
Moreover, our results showed that although the expression of PER-1 did not show significant differences in the morning and night before and after GCSF injection. In addition to, PER-2 expression did not show any significant changes during the study. Besides, there were not any significant correlation between the PER-1 or PER-2 expression and CD34+ cell counts. Therefore, it could be concluded that, PER-1 and PER-2 expression has not significant effect on the blood circulating cell count. But the expression level of both PER-3 and CRY-1 was higher in the morning than in the night in both control and target group. So it can be stated that the co-ordination between these two genes leads to an increased rate of mobilization in the early hours of the day than at night. And G-CSF injection has no effect on gene expression pattern.
Conclusion
Our findings indicated that the clock genes including PER-1 and PER-2 have not any significant roles in mobilization of HSCs from bone marrow to peripheral blood, but PER-3 and CRY-1 have a direct impact.
Declarations
Compliance with ethical standards, conflict of interest: The authors declare that they have no conflict of interest.
Ethical: All procedures have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent: Informed consent was assessed prior to intervention. Details that disclose the identity of the subjects under study were omitted.
Author contributions: SA, AF: Conception and design, acquisition of data; SA: Drafting the article; ER: analysis and interpretation of data and revising the draft; A.H. Supervising and editing. All authors revised final approval of the version to be submitted for publication.
Acknowledgements: We wish to thank all our colleagues in Taleghani Hematopoietic stem cell transplantation center, Tehran, Iran.
References
- Khosravi A, Shahrabi S, Shahjahani M, Saki N. The bone marrow metastasis niche in retinoblastoma. Cellular Oncology. 2015; 38(4): 253-63.
- Pelus LM. Peripheral blood stem cell mobilization: New regimens, new cells, where do we stand. Current opinion in hematology. 2008; 15(4): 285.
- Gillette JM, Lippincott-Schwartz J. Hematopoietic progenitor cells regulate their niche microenvironment through a novel mechanism of cell-cell communication. Commun Integr Biol. 2009; 2(4): 305: 7.
- Montgomery M, Cottler-Fox M. Mobilization and collection of autologous hematopoietic progenitor/stem cells. Clin Adv Hematol Oncol. 2007; 5(2): 127-36.
- Méndez Ferrer S, Battista M, Frenette P. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008; 452(7186): 442-7.
- Méndez-Ferrer S, Frenette P. Cooperation of beta (2) and beta (3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010; 139-44:1192.
- Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008; 3(5): 486-92.
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006; 124(2): 407-21.
- Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2003; 23(1): 1-25.
- Hida A, S Kitamura, K Mishima. Pathophysiology and pathogenesis of circadian rhythm sleep disorders. Journal of physiological anthropology. 2012; 31(1): 7.
- Brown SA, E Kowalska, R Dallmann, (Re) inventing the circadian feedback loop. Developmental cell. 2012; 22(3): 477-487.
- Robinson I, A Reddy. Molecular mechanisms of the circadian clockwork in mammals. FEBS letters. 2014; 588(15): 2477-2483.
- Ray S, AB Reddy. Cross‐talk between circadian clocks, sleep‐wake cycles, and metabolic networks: Dispelling the darkness. BioEssays. 2016; 38(4): 394-405.
- Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, et al. Non hematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004; 104(12): 3581-7.
- Crews ST, Thomas JB, Goodman CS. The Drosophila single-minded gene encodes a nuclear protein with sequence similarity to the per gene product. Cell. 1988; 52(1): 143-51.
- Jansen J, Hanks S, Thompson JM, Dugan MJ, Akard LP. Transplantation of hematopoietic stem cells from the peripheral blood. Journal of cellular and molecular medicine. 2005; 9(1): 37-50.
- Klarsfeld A, Malpel S, Michard-Vanhée C, Picot M, Chélot E, et al. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. Journal of Neuroscience. 2004; 24(6): 1468-77.
- Aschoff J. Circadian control of body temperature. Journal of thermal Biology. 1983; 8(1-2): 143-7.
- Cajochen C, Kräuchi K, Wirz‐Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. Journal of neuroendocrinology. 2003; 15(4): 432-7.
- Brown SA, Azzi A. Peripheral circadian oscillators in mammals. Circadian clocks: Springer; 2013; 45-66.
- Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, et al. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003; 302(5643): 255-9.
- Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nature reviews Immunology. 2013; 13(3): 190-8.
- Laerum OD. Hematopoiesis occurs in rhythms. Exp Hematol. 1995; 23(11): 1145-7.