Research Article
Volume 3, Issue 5
Chailing Decoction Generated Foxp3+ Regulatory T Cells and Suppressed Activity of mTOR Signaling Pathway in a Murine Cardiac Transplantation Model
Deli Cui1 ; Youman Zhang2 ; Changjun Tan3 ; Zhong Liu4*
1Department of General Surgery, Tianjin Children’s Hospital, Tianjin 300074, China.
2Department of General Surgery, Ankang Central Hospital, Ankang, Shanxi 725000, China.
3Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, 200032, China.
4Department of General Surgery, Shenzhen University General Hospital/Shenzhen University Clinical Medical Academy, Shenzhen, Guangdong, 518055, China.
Corresponding Author :
Zhong Liu
Tel: +8618098875979;
Email: liuzhong5979@szu.edu.cn
Received : Apr 17, 2024 Accepted : May 16, 2024 Published : May 23, 2024 Archived : www.meddiscoveries.org
Citation: Cui D, Zhang Y, Tan C, Liu Z. Chailing Decoction Generated Foxp3+ Regulatory T Cells and Suppressed Activity of mTOR Signaling Pathway in a Murine Cardiac Transplantation Model. Med Discoveries. 2024; 3(5): 1157.
Copyright: © 2024 Liu Z. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objectives: To investigate the effects of Chailing Decoction, a herbal medicine, on the generation of regulatory T cells (Tregs) and its potential signaling pathways in preventing graft rejection.
Methods: The study utilized C3H mice that underwent cardiac transplantation and received either Chailing Decoction or rapamycin. The survival time of the cardiac allografts, histopathological changes, the number of Tregs, and the expression of the mammalian Target of Rapamycin (mTOR) signaling pathway were evaluated.
Results: The results demonstrated that treatment with Chailing Decoction significantly prolonged the survival time of the cardiac allografts. Histological analysis revealed reduced inflammatory infiltration in the allografts following Chailing Decoction treatment. The percentage and absolute number of Tregs were also increased after treatment. Additionally, Chailing Decoction treatment effectively inhibited the activity of the mTOR signaling pathway, as indicated by decreased phosphorylation levels of Akt, mTOR, p70S6k, and 4EBP1. Chailing Decoction treatment induced hyporesponsiveness to fully allogeneic cardiac grafts and promoted the generation of Tregs. These effects may be attributed, at least in part, to the modulation of the mTOR signaling pathway.
Conclusion: These findings suggest that Chailing Decoction has potential as a therapeutic intervention to prevent graft rejection and promote immune tolerance in transplantation.
Keywords: Chailing Decoction; Cardiac transplantation; Regulatory T cells; mTOR pathway.
Introduction
Transplantation is the ultimate treatment for patients with total loss of function of a life-sustaining organ. The main therapy for allograft rejection is immunosuppressive drugs [1]. However, non-specific immunosuppression mainly causes numerous side effects, such as opportunistic infection and cancer [2]. Thus, reducing the use of immunosuppressive drugs and inducing donor-specific tolerance are the main objectives in transplantation. Regulatory T cells (Tregs) [3] were considered to be essential for the induction and maintenance of peripheral tolerance. A previous study [4] demonstrated the ability of Tregs to delay/prevent graft rejection by inducing donor-specific tolerance. Therefore, identifying agents that promote the generation of Tregs may have practical implications for developing new tolerance strategies in transplantation. Herbal medicines, including Chailing Decoction (in Chinese)/Sairei-to (in Japanese), have long been used as an alternative therapy in China, Japan, and Korea, and have recently become popular in Europe [5] and the United States [6] because they can provide health benefits. Chailing Decoction is composed of 12 different herbs, in which some components, such as saikosaponin, baicalein, and glycyrrhizic acid, have inhibitory effects on the mammalian Target of Rapamycin (mTOR) signaling pathway. Rapamycin is a well-known macrolide immunosuppressant that inhibits the mTOR protein kinase [7], exerting immunosuppressive effects by promoting an increase in immunosuppressive Tregs [8]. We have previously reported that administration of Chailing Decoction prolongs allograft survival and generates Tregs [9]. However, the mechanism of generating Tregs by Chailing Decoction has not been clarified yet. In the present study, we assessed the possible signaling pathway of Chailing Decoction on the alloimmune response in a murine model of cardiac allograft transplantation.
Materials and methods
Animal welfare statement
Male C57BL/6 (H-2b ) and C3H/He (H-2k) mice (age, 8-12-weekold) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China), housed in conventional facilities at the Biomedical Services Unit of Zhongshan Hospital Affiliated to Fudan University (Shanghai, China), and used in accordance with the guidelines for animal experiments approved by the Animal Use and Care Committee of Fudan University.
Cardiac transplantation
All transplant procedures were performed on mice under general anesthesia. Fully vascularized heterotopic hearts from C57BL/6 donor mice were transplanted into C3H mice using microsurgical techniques [10]. Postoperatively, graft function was assessed daily by palpation for the evidence of contraction. Rejection was defined as complete cessation of the heartbeat and confirmed by direct visualization and histological examination of the graft.
Treatment with Chailing Decoction or rapamycin
C3H recipients of C57BL/6 hearts were given distilled water (control group), in which 1.5 mg/kg/day of rapamycin (Monmouth Junction, NJ, USA) or 2 g/kg/day of Chailing Decoction (Tsumura, Tokyo, Japan) were given based on their standard dosage regimens from the day of transplantation to the next 7 days. The medicine was dissolved in distilled water and given orally using a metal tube (Thomas Scientific, Swedesboro, NJ, USA).
Histological examinations of harvested grafts
Cardiac allografts were removed on 3, 7, or 14 days after transplantation and studied histologically. Using routine procedures, the specimens were immersed in 5% neutrally buffered formalin and embedded into paraffin. Paraffin-embedded sections (thickness, 4 mm) were cut, mounted on saline-coated slides, and stained with Hematoxylin and Eosin (HE). For the HE staining, firstly, the slides were placed with a section in a metal staining rack, immersed in Harris hematoxylin solution for 10 sec, and the rack of a beaker was removed with tap water. Then, sections were immersed in eosin for 30 sec and the rack of a beaker was removed with tap water again. Finally, sections were dehydrated in ascending alcohol solutions, cleared with xylene, placed on a clean glass slide, and a clean coverslip was mounted over the section using paramount. Transplant vasculopathy was assessed after Elastica-van Gieson staining.
Immunofluorescence staining
The specimens of cardiac grafts were fixed with 4% paraformaldehyde for 10 min at room temperature, followed by treatment with membrane penetration solution (0.3% Triton-100) for 10 min at room temperature. The specimens were washed with Phosphate-Buffered Saline (PBS) five times, and then, incubated with mouse monoclonal anti-CD4 antibody (1:100; sc19643; Santa Cruz Biotechnology Inc., Dallas, TX, USA) for 1 h at 37°C. Afterwards, they were incubated with an appropriate secondary antibody (FITC-conjugated goat anti-rabbit IgG antibody (1:80; F1262; Sigma-Aldrich, St. Louis, MO, USA)) for localization of CD4 for 45 min at 37°C. For forkhead, winged-helix transcription factor (Foxp3, a specific marker of natural Tregs), permeabilization was carried out with 0.4% Triton X-100 for 45 min, followed by incubation with mouse monoclonal anti-Foxp3 antibody (1:30; sc-53876; Santa Cruz Biotechnology Inc.), and with TRITC-conjugated goat anti-mouse IgG antibody (1:50; T7782; Sigma-Aldrich) for localization of Foxp3 markers. After thrice washing with PBS, samples were mounted in a mounting medium (M1289; Sigma-Aldrich), observed under a Zeiss fluorescence microscope (Carl Zeiss, Oberkochen, Germany), and image analysis was performed using Zeiss LSM 510 software.
Flow cytometry analysis
The expression levels of CD4, CD25, and Foxp3 in splenocytes were determined by flow cytometry. At 3 or 7, or 14 days after transplantation, splenocytes from recipients were stained with fluorochrome-conjugated anti-CD4 and anti-CD25 monoclonal antibodies (mAb; RM4-5 and PC61, respectively; BD Biosciences, San Jose, CA, USA), and anti-mouse Foxp3 mAb antibody (FJK-16s; eBioscience, San Diego, CA, USA), as well as their isotype controls. The stained cells were analyzed using a flow cytometer (FACSCalibur; BD Biosciences).
Mixed leukocyte culture
The responder cells were splenocytes obtained from naive C3H mice or untreated rapamycin-treated or Chailing Decoction-treated C3H mice that had undergone transplantation with C57BL/6 hearts. The stimulator cells were splenocytes obtained from C57BL/6 mice. Then, the responder cells and stimulator cells were cultured in a Roswell Park Memorial Institute (RPMI)- 1640 medium, containing 10% (v/v) fetal bovine serum (FBS; Gibco, New York, NY, USA) and 1% (v/v) penicillin/streptomycin (Gibco) at 37°C in a humidified incubator with 5% Carbon Dioxide (CO2 ). Cellular proliferation was assessed by an EnzymeLinked Immunosorbent Assay (ELISA) kit for bromodeoxyuridine (BrdU) incorporation.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR
Total RNA was isolated from fresh-frozen spleen tissues using TRIzol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. Total RNA (0.5 μg) was reversely transcribed into cDNA with a MaximaTM H Minus cDNA Synthesis Master Mix kit (Thermo Fisher Scientific, Waltham, MA, USA). The qRT-PCR was performed on a 7500 fast real-time PCR platform (Applied Biosystems, Foster City, CA, USA) using a Power SYBR-Green Master Mix kit (Applied Biosystems) to detect the expression levels of Foxp3, mTOR, AKT, P70s6k, and 4E-BP1. Data were normalized to the expression of Glyceraldehyde-3- Phosphate Dehydrogenase (GAPDH) as an internal loading control. The PCR primers are shown in Table 1. The PCR was denatured at 95°C for 5 sec; annealing was conducted at 55°C for 30 sec and elongation at 65°C for 20 sec for 40 cycles.
Western blot analysis
Total protein was extracted from spleen tissues using the Radio-Immunoprecipitation Assay (RIPA) lysis buffer. Herein, proteins (100 mg) were subjected to Sodium Dodecyl SulfatePolyacrylamide Gel Electrophoresis (SDS-PAGE), and then, transferred on to Polyvinylidene Difluoride (PVDF) membranes (Bio-Rad Laboratories Inc., Hercules, CA, USA). Immunoblotting was then performed using phospho-p70S6K T389 (1:1000), p70S6K (1:1000), phospho-Akt S473 (1:2000), Akt (1:2000), phospho-4E-BP1 S65 (1:1000), 4EBP1 (1:1000), phospho-mTOR (1:1000), mTOR (1:1000), GAPDH (1:2000), and secondary HRPconjugated (1:10,000) antibodies. Immunoreactivity was then detected by an enhanced chemiluminescence kit (Pierce, Rockford, IL, USA), according to the manufacturer’s instructions. Protein bands were then quantified using a calibrated Bio-Rad Image Lab densitometer (Bio-Rad Laboratories Inc.), and they were expressed as band intensity of phosphorylated proteins normalized to the relevant total proteins.
Statistical analysis
Cardiac allograft survival of mice was compared using the Mann-Whitney U test. In the flow cytometry, RT-PCR, and Western blot assays, two groups were compared using unpaired Student’s t-test. GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA) was used to perform statistical analysis. P<0.05 was considered statistically significant.
Results
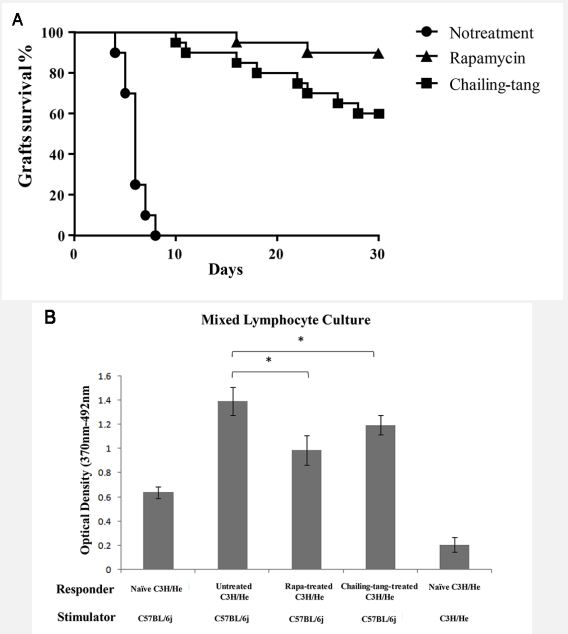
Chailing Decoction treatment prolonged the survival time of allograft after cardiac transplantation
The C3H mice given distilled water rejected C57BL/6 cardiac allografts acutely (Median Survival Time [MST] 7 days). The C3H mice that received rapamycin had a prolonged graft survival (MST>30 days; P<0.01 vs. distilled water-treated controls). The C3H recipients treated with Chailing Decoction also had a prolonged graft survival (MST>30 days; P<0.01 vs. distilled watertreated controls). But there were no difference between the group of Chailing Decoction treatment and the group of rapamycin treatment (Figure 1A). Proliferation of splenocytes from C3H transplant recipients that received Chailing Decoction was markedly suppressed compared with that of splenocytes from untreated C3H recipients, which was similar to the mixed lymphocytes treated with rapamycin (Figure 1B). These results suggested that Chailing Decoction treatment could induce hyporesponsiveness to fully mismatched cardiac allografts.
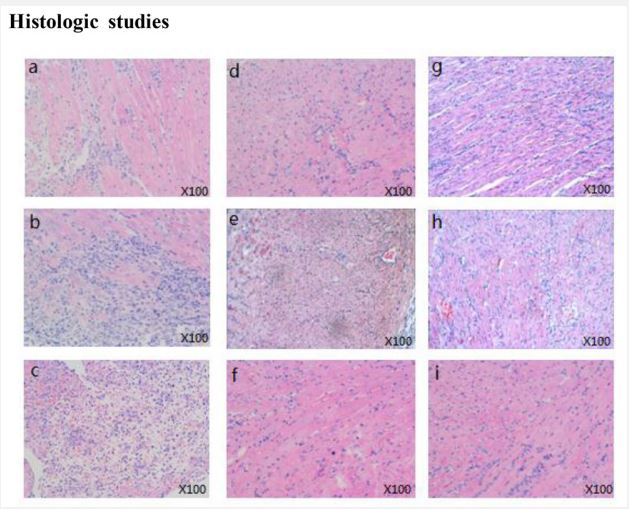
Preserved graft structure of cardiac allografts in transplant recipients after treatment with Chailing Decoction
In order to indicate whether Chailing Decoction treatment of C3H recipients can protect the graft structure or not, histological examinations of cardiac allograft samples were carried out 3, 7, or 14 days after transplantation. Samples from untreated recipients showed myocyte damage, edema, and aggressive inflammatory infiltrates in the acute rejection process (Figure 2A-C). In contrast, cardiac allografts in transplant recipients that received rapamycin (Figure 2D-F) or Chailing Decoction (Figure 2G-I) preserved graft structure with few myocardial injuries and infiltrating leukocytes.
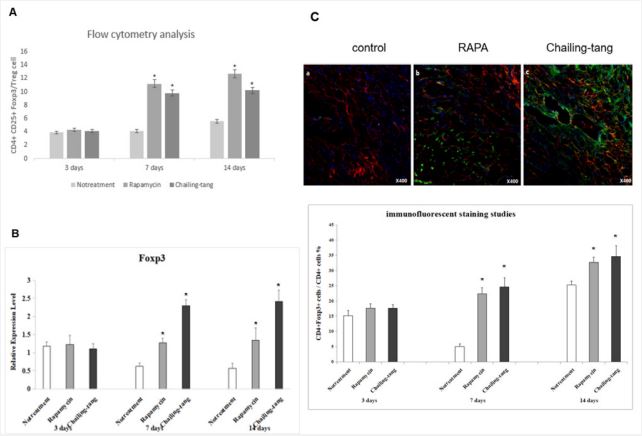
Chailing Decoction treatment significantly increased the number of tregs in the cardiac transplantation recipients
To illustrate whether the application of Chailing Decoction can increase the number of Tregs or not, flow cytometry, qRTPCR, and immunofluorescence staining of Tregs were performed on 3, 7, or 14 days after transplantation. The results of flow cytometry showed that the number of CD4+ CD25+ Foxp3+ cells increased in the splenocytes of recipients treated with Chailing Decoction or rapamycin compared with that of untreated C3H recipients (Figure 3A). The results of qRT-PCR revealed that the expression level of Foxp3 was upregulated in the splenocytes of recipients treated with Chailing Decoction or rapamycin compared with that of untreated C3H recipients (Figure 3B). In addition, the immunofluorescence staining of grafts showed that the number of Foxp3+ Tregs was remarkably higher in recipients that were treated with Chailing Decoction or rapamycin than the grafts from the untreated recipients (Figure 3C).
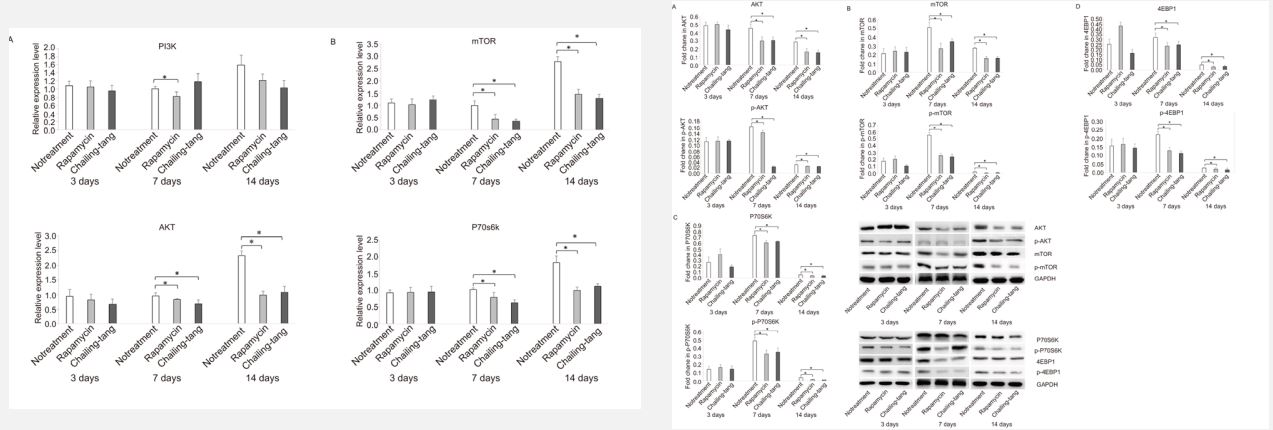
Chailing Decoction treatment inhibited molecular expression of mTOR pathway in cardiac transplantation recipients
To determine whether the effects of Chailing Decoction on Tregs in cardiac transplanted mice were associated with the mTOR pathway or not, the molecular expression of mTOR pathway was detected by qRT-PCR and Western blotting. The results of qRT-PCR revealed that the expression levels of Akt, mTOR, and P70s6k were significantly reduced in the splenocytes of cardiac transplanted mice treated with Chailing Decoction compared with those in untreated mice, similar to the rapamycin-treated mice (Figure 4-1). The results of Western blotting showed that the phosphorylation levels of Akt, mTOR, P70s6k, and 4E-BP1 were significantly downregulated in the splenocytes of cardiac transplanted mice treated with Chailing Decoction compared with those in untreated mice, similar to the rapamycin-treated mice (Figure 4-2).
Discussion
In the present study, to describe the possible signaling pathways of Chailing Decoction on the generation of Tregs to prevent an alloimmune response, C3H mice underwent transplantation of C57BL/6 hearts, and they received either oral Chailing Decoction or rapamycin post transplantation. The results shown that Chailing Decoction treatment could prolong the survival time of cardiac allografts, and the inflammatory infiltration in cardiac grafts was significantly reduced. In addition, Chailing Decoction treatment increased the number of Foxp3+ Tregs and inhibited the activity of the mTOR pathway molecules.
Transplantation is the ultimate treatment for patients with total loss of function of a life-sustaining organ [11]. The main therapy for allograft rejection is immunosuppressive drugs, however, they mainly have toxicities and exhibited adverse effects [12], such as opportunistic infection and cancer [13]. Herbal medicines, including Chailing Decoction (in Chinese)/Sairei-to (in Japanese), have long been used as an alternative therapy in China, Japan, and Korea, and have recently become popular in Europe [5] and the United States [6] because they can provide health benefits. Chailing Decoction was reported to effectively treat immune rejection in cardiac transplantation, without the above-mentioned adverse effects. Chailing Decoction is composed of 12 different herbs: bupleuri radix, scutellariae radix, pinelliae tuber, ginseng radix, glycyrrhizae radix, zingiberis rhizome, zizyphi fructus, polyporus sclerotium, poria sclerotium, atractylodis lanceae rhizoma, alismatis rhizome, and cinnamomi cortex. We have previously reported that administration of Chailing Decoction could prolong allograft survival and generate Foxp3+Tregs [14]. Foxp3 is known as the most specific marker distinguishing regulatory T cells from activated CD4+ effector T cells, previous studies showed that the PI3K-Akt-mTOR signaling pathway negatively regulated Foxp3 expression [15], and specifically prevented the induction of Foxp3 [16]. Transient TCR stimulation drove PI3K-Akt-mTOR signaling that antagonized Foxp3 expression. Thus, selective blockade of PI3K–Akt–mTOR signaling pathway would augment Foxp3 expression. Rapamycin (sirolimus) is a well-known macrolide immunosuppressant that inhibits the mTOR protein kinase [17]. Previous studies showed that rapamycin exerted immunosuppressive effects by increasing the number of immunosuppressive Tregs [18]. Some herbal components of Chailing Decoction, such as saikosaponin, baicalein, and glycyrrhizic acid, have been demonstrated to exert inhibitory effects on the mTOR signaling pathway. In the present study, we found that Chailing Decoction treatment markedly suppressed the mTOR signaling pathway molecules, as phosphorylation of the mTOR downstream targets, p70S6K and 4EBP1, which is consistent with the role of rapamycin. P70S6K and 4E-BP1 are well-characterized substrates for mTORC1 [19], one of the protein complexes of mTOR. The results indicated that Chailing Decoction treatment could effectively block mTORC1. It is noteworthy that mTORC1 controls protein translation, autophagy, and other cellular processes through the phosphorylation of substrates that include p70S6K and 4E-BP1 [20]. We, in the present study, found that Chailing Decoction treatment downregulated the activation of Akt, mTOR, p70S6K, and 4EBP1, while increased the number of Foxp3+ Tregs. These results revealed that Chailing Decoction could upregulate Foxp3+ Tregs by blocking the activity of the PI3K-Akt-mTOR signaling pathway, which is consistent with the role of rapamycin.
As a limitation of the present study, due to the multi-component and multi-target of the Chailing Decoction, uncontrollable composition is associated with a disadvantage. However, this was the first study on the Chailing Decoction for regulating intracellular signaling pathways. Therefore, further researches should be carried out to isolate the components of the Chailing Decoction to find out the best activity and target with extended experimental plans.
Conclusion
In summary, the administration of Chailing Decoction could inhibit mice cardiac allograft rejection by increasing the number of Foxp3+ Tregs. The underlying mechanism was found to be associated with the suppression of the mTOR signaling pathway.
Declarations
Funding: This work was supported by the National Natural Science Foundation of China (No. 81373874 and 81973664), the Natural Science Foundation of Shenzhen Science and Technology Innovation Commission (JCYJ20190808140601638).
Author contributions: Deli Cui and Zhong Liu conceived and supervised the study; Deli Cui and Zhong Liu designed experiments; Deli Cui, Changjun Tan and Youman Zhang performed experiments; Deli Cui and Zhong Liu analysed data; Deli Cui and Zhong Liu wrote the manuscript; Changjun Tan and Zhong Liu made manuscript revisions. All authors reviewed the results and approved the final version of the manuscript.
Conflict of interest: The authors report there are no competing interests to declare.
Acknowledgement: None.
References
- H Parlakpinar, M Gunata. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs, Immunopharmacology and Immunotoxicology. 2021; 43(6): 651-65.
- N Yilmaz, R Sari, G Suleymanlar, S Ozdem. Effects of Different Immunosuppressive Drugs on Incretins in Renal Transplant Patients, Journal of the National Medical Association. 2020; 112(3): 250-57.
- FC Edozie, EA Nova-Lamperti, GAM Povoleri, C Scottà, S John, et al. Regulatory T-Cell Therapy in the Induction of Transplant Tolerance, Transplantation. 2014; 98(4): 370-379.
- M Romano, G Fanelli, CJ Albany, G Giganti, G Lombardi. Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity, Frontiers in immunology. 2019; 10: 43.
- E van Galen. Traditional herbal medicines worldwide, from reappraisal to assessment in Europe, Journal of Ethnopharmacology. 2014; 158: 498-502.
- EA Frost. Herbal medicines and interactions with anesthetic agents, Middle East J Anaesthesiol.2006; 18(5): 851-78.
- D Wang, HJ Eisen. Mechanistic Target of Rapamycin (mTOR) Inhibitors, Handbook of Experimental Pharmacology, Springer Berlin Heidelberg. 2021.
- X Chen, S Li, D Long, J Shan, Y Li. Rapamycin facilitates differentiation of regulatory T cells via enhancement of oxidative phosphorylation, Cellular Immunology. 2021; 365: 104378.
- Q Zhang, D Iwami, O Aramaki, S Yakubo, K Nishimura, et al. Prolonged Survival of Fully Mismatched Cardiac Allografts and Generation of Regulatory Cells by Sairei-to, a Japanese Herbal Medicine, Transplantation. 2009; 87(12) : 1787-91.
- D Cui, C Tan, Z Liu. An alternative technique of arterial anastomosis in mouse heart transplantation, Clinical Transplantation.2018; 32(6): e13264.
- N Strasser, S Gruber, K Hoetzenecker, W Klepetko, Z Szépfalusi, et al. Quality of Life after Pediatric Lung Transplantation, The Journal of Heart and Lung Transplantation. 2020; 39(4): S464.
- JC Olson. Immunosuppressive drugs and associated complications in abdominal organ transplantation, Current Opinion in Critical Care. 2022; 28(2): 208-15.
- MB Roberts, JA Fishman. Immunosuppressive Agents and Infectious Risk in Transplantation: Managing the Net State of Immunosuppression, Clinical infectious diseases: An official publication of the Infectious Diseases Society of America. 2021; 73(7): e1302-17.
- T Kaburaki, Q Zhang, X Jin, M Uchiyama, Y Fujino, et al. Effects of Japanese herbal medicine Sairei-to on murine experimental autoimmune uveitis, Graefe’s Archive for Clinical and Experimental Ophthalmology. 2013; 251(12): 2733-9.
- B Lamarthée, A Marchal, S Charbonnier, T Blein, J Leon, et al. Transient mTOR inhibition rescues 4-1BB CAR-Tregs from tonic signal-induced dysfunction, Nature communications. 2021; 12(1): 6446 Copyright © 2024 Liu Z. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Y Shao, WY Yang, F Saaoud, Ct Drummer, Y Sun, et al. IL-35 promotes CD4+Foxp3+ Tregs and inhibits atherosclerosis via maintaining CCR5-amplified Treg-suppressive mechanisms, JCI insight. 2021; 6(19) : e152511.
- U Saran, M Foti, JF Dufour. Cellular and molecular effects of the mTOR inhibitor everolimus, Clinical Science. 2015; 129(10): 895-914.
- M Battaglia, A Stabilini, MG Roncarolo. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells, Blood. 2005; 105(12): 4743-8.
- E Villa, U Sahu, BP O’Hara, ES Ali, KA Helmin, et al. mTORC1 stimulates cell growth through SAM synthesis and m(6)A mRNAdependent control of protein synthesis, Molecular cell.2021; 81(10): 2076-93.
- R Böhm, S Imseng, RP Jakob, MN Hall, T Maier, et al. The dynamic mechanism of 4E-BP1 recognition and phosphorylation by mTORC1, Molecular Cell. 2021; 81(11): 2403-16.