Research Article
Volume 3, Issue 4
Evaluation of the Antimicrobial and Antioxidant Activity of the Pulp of Artocarpus heterophyllus Lam (Moraceae)
Johnson-Ajinwo*; Okiemute Rosa*; Paul-Worika; Priscilla Nengi
Department of Pharmaceutical/Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Nigeria.
Corresponding Author :
Johnson - Ajinwo & Okiemute Rosa
Email: worikap@gmail.com & okiemute_2002@yahoo.co.u
Received : Mar 15, 2024 Accepted : Apr 29, 2024 Published : Apr 30, 2024 Archived : www.meddiscoveries.org
Citation: Ajinwo J, Rosa O, Worika P, Nengi P. Evaluation of the Antimicrobial and Antioxidant Activity of the Pulp of Artocarpus heterophyllus Lam (Moraceae). Med Discoveries. 2024; 3(4): 1148.
Copyright: © 2024 Okiemute Rosa. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Artocarpus heterophyllus Lam. (Moraceae) - Jackfruit beyond its culinary uses has been used to treat ailments ethnomedicinally. Its bark, leaves, seeds, and pulp are rich sources of bioactive compounds. This research aims to evaluate the antimicrobial and antioxidant activity of the pulp of the fruit; to identify the bioactive compounds present, that contributes these activities. The pulverised dried pulp was extracted using dichloromethane and methanol (1:1) and partitioned into respective fractions using n-hexane and methanol. Proximate analysis and Phytochemical screening were carried out using standard methods. Gas Chromatography - Mass Spectrometry (GC-MS) and Fourier Transform Infrared Spectroscopy (FTIR) were used for chemical profiling and detection of functional groups respectively. Antimicrobial activity testing was carried out, using the agar well diffusion assay. Antioxidant activity was evaluated using DPPH (2,2-diphenyl-1-picrylhydrazyl) assay. Proximate analysis showed the presence of moisture (16.96%), fibre (20.89%), carbohydrate (50.63%), protein (7.20%), ash (2.26%), and lipid (2.06%). The phytochemical screening revealed the presence of alkaloids, flavonoids, phenolics, saponins, carbohydrates, proteins, tannins, steroids/triterpenoids and phlobatannins. Ten compounds were identified from the GC-MS Analysis. The pharmacologically active compounds were 9,12-octadecadienoic acid methyl ester, 9,12,15-octadecatrienoic acid methyl ester, 1-docosene, and lycopersene. FTIR analysis revealed the presence of aliphatic hydrocarbons (alkane, alkene) and oxygenated functional groups (hydroxyl, esters, aldehydes, ketones, ethers). The methanol, n-hexane fractions and total extract exhibited antimicrobial activity against Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli. Regarding antioxidant activity, the methanol, n-hexane fractions and total extract showed IC50 values of 39.191 µg/ml, 102.5 µg/ml and 70.10 µg/ml respectively. The results of this study demonstrates that Artocarpus heterophyllus Lam. possesses significant antimicrobial and antioxidant properties due to the presence of bioactive compounds and holds promising implications for pharmaceutical applications.
Introduction
Natural products serve as a repository for bioactive compounds that have developed over millions of years to shield plants from environmental stress and pathogens. These compounds can range from molecules such as vitamins to intricate structures like proteins and enzymes, playing a vital role in the organism’s ability to survive and adapt to its surroundings. Natural products have been used for centuries in traditional medicine systems worldwide for treating various ailments and they serve as important leads for developing new and more effective drugs against modern diseases.
Contemporary medicine is presently dealing with the challenge of Antimicrobial Resistance (AMR) and diseases associated with Oxidative Stress. Antimicrobial Resistance (AMR) denotes the capacity of microorganisms, encompassing bacteria, viruses, fungi, and parasites, to adapt and resist the effects of medications intended to eliminate or impede their growth. This resistance renders previously effective antimicrobial drugs, such as antibiotics, antivirals, antifungals, and antiparasitic medications, ineffective. Antimicrobial resistance poses a pressing global public health danger, causing a minimum of 1.27 million fatalities worldwide and being linked to nearly 5 million deaths in 2019. In the United States, over 2.8 million infections resistant to antimicrobials emerge annually, resulting in the death of more than 35,000 individuals, as reported by the CDC’s 2019 Antibiotic Resistance (AR) Threats Report [1].
Oxidative stress is characterized by a disparity between the generation of Reactive Oxygen Species (ROS) and the body’s capacity to neutralize or eliminate their detrimental effects via antioxidant mechanisms. Reactive Oxygen Species (ROS) naturally arise as inevitable by-products of metabolic activities. In the course of metabolic processes, these radicals serve as mediators facilitating the transfer of electrons in diverse biochemical reactions. The continuous production of free radicals during the metabolic processes culminates in the development of antioxidant defence mechanisms. These are intended to limit the intracellular levels of these reactive species and control the occurrence of damage caused by them. However, an imbalance between the production of these free radicals and their mop up by the body, can lead to damage at the cellular and molecular levels, contributing to the development and progression of various diseases including cardiovascular diseases, cancer, diabetes, chronic inflammatory diseases, aging and age-related diseases, among others.
In a world grappling with the dire consequences of AMR and oxidative stress-related diseases, and as conventional treatments face diminishing effectiveness, researchers are turning their attention to nature’s pharmacy in search of alternative solutions. The exploration of natural products like Jackfruit signifies a ray of hope.
Artocarpus heterophyllus Lam. (Moraceae), commonly known as jackfruit, is one of the most useful fruits of the tropics. It is unarguably regarded as the largest edible fruit in the world with weight of individual fruits ranging from 5 to 50 kg. Its sweet taste and exotic flavour positions it as the sweetest and most delicious tropical fruit. Jackfruit grows in warm and moist regions. It is found in many parts of Asia, Africa, and South America. Significantly, in Nigeria, it has been found in the Southern region, including states like Rivers and Akwa Ibom, and is easily available for purchase at local fruit markets. Jackfruit trees stand out due to their remarkable yield, with a high productivity of 25.71 tonnes per hectare (t/ha). This translates to 25,710 kilograms of fruit per 10,000 square meters, far exceeding the typical 5-15 t/ha of other fruit trees. A single mature jackfruit tree can bear an impressive 10 to 200 fruits, contributing significantly to its global demand and income generation.
Jackfruit - Artocarpus heterophyllus Lam. (Moraceae) has been utilized in traditional practices for centuries, serving a variety of purposes beyond its culinary uses. In traditional medicine systems, various parts of the jackfruit tree have been used to treat ailments. The latex from the tree has been applied to wounds for its antiseptic properties. Jackfruit leaves were used to make poultices for skin issues and to help alleviate fever. The root extract was used as a remedy for skin diseases. The jackfruit’s seeds are known for their digestive benefits. They are often roasted or boiled and consumed to aid in digestion and alleviate constipation. In some cultures, jackfruit seeds have been used as a natural remedy to regulate blood sugar levels. The seeds are believed to have properties that can help manage diabetes. The leaves of the jackfruit tree are known for their anti-inflammatory properties. They are used to make poultices to reduce inflammation and pain in certain conditions. Jackfruit leaves were traditionally used to provide relief from asthma symptoms. The leaves were boiled, and the steam was inhaled for respiratory relief. The latex derived from the jackfruit tree has been used as a natural adhesive in various applications, including bookbinding, handicrafts, and even as an ingredient in culinary preparations. Some traditional remedies included jackfruit as a component in face masks and skin treatments due to its perceived ability to promote youthful and glowing skin [2].
Jackfruit has a wide range of beneficial biological activities, including antimicrobial, antioxidant, antidiabetic, and anti-inflammatory properties. It’s an abundant source of magnesium and potassium, crucial for bone health, blood pressure regulation, and overall cardiovascular function. It’s also a good source of Vitamin C, vital for skin health and protection against aging and sun damage. Additionally, it contains Carotenoids, offering valuable support against chronic diseases like cancer, inflammation, and age-related conditions.
Much studies on this superfruit, has focussed on the leaves, bark and seeds, with scant report on the pulp, which is heavily consumed. Also, there are very few studies carried out on the Nigerian species, which have undermined the value and benefits of Jackfruit in the country. Since the antimicrobial and antioxidant potential inherent in the pulp of Artocarpus heterophyllus Lam. remain largely unexplored. This work aims to elevate Jackfruit from being just a cultural remedy to being an evidence-based solution through scientific exploration.
Material and methods
Collection of plant materials
The sample, Artocarpus heterophyllus Lam. (Moraceae), commonly known as Jackfruit was harvested from Khana Local Government Area (LGA) of River State. The sample was identified and authenticated procedures conducted by a Taxonomist, Dr. Suleiman Mikailu, of the Department of Pharmacognosy and Phytotherapy, Faculty of Pharmaceutical Sciences, University of Port-Harcourt. The Voucher Specimen Number is UPHM0603.
Materials and apparatus
Grinding machine, weighing balance, measuring cylinders, wide mouth bottles, spatula, foils, rotary evaporator, water bath, desiccator, airtight container, separating funnel etc.
Drugs, chemicals and reagents
Dichloromethane (Sigma-Aldrich brand), methanol (SigmaAldrich brand), water, n-hexane, Diphenyl picryl hydralazine, Dimethyl sulfoxide (Sigma-Aldrich brand), hydrochloric acid, magnesium metal, sodium hydroxide, ferric chloride, dragendorff’s reagent, wagner’s reagent, olive oil, hydrochloride acid, chloroform, acetic anhydride, sulphuric acid, Fehling’s solution A + B, molisch reagent, million’s reagent, glacial acetic acid, picric acid solution, distilled water.
Sample preparation
The jackfruits were washed properly and carefully sliced open using clean knives, with the pulp separated from the seeds. To ensure there was no leftover debris, the obtained pulp was again washed. The pulp was sun dried for a week. The dried jackfruit pulp was ground into a fine powder using a grinder after it reached the required level of dryness. After being finely ground, the sample was carefully kept in glass containers that were airtight for subsequent utilization.
Physicochemical analysis
Determination of moisture content: The fresh jackfruit pulp was accurately weighed, and the initial weight was recorded. The pulp was placed in a pre-heated oven at a specified temperature typically 70-80°C. It was allowed to dry until a constant weight was achieved, typically over a period of 24 hours. The sample was removed from the oven and allowed to cool. The final weight after drying was recorded.
The moisture content was calculated using the formula:
Moisture Content % = (Initial weight - Final weight)/(Initial weight) ×100
Determination of pH: The pH meter’s electrode assembly was immersed in a clean and dry beaker containing a standard buffer solution with a pH of 9. After rinsing with distilled water, the electrode assembly was placed into a solution with a standard pH of 4 and finetuned to the desired pH using the asymmetry potential knob. Following this adjustment, the electrode assembly was lifted, washed twice with distilled water, and subsequently immersed in the juice extracted from fruit pulp to record the pH using the meter.
Proximate analysis
The Proximate analysis was carried out on the pre-dried plant sample. This was necessary as the aim was to obtain the nutritive constituents of the plant without interference of much moisture.
Determination of moisture content: One gram of the sample was accurately weighed into a clean, dried porcelain evaporating dish. The dish, containing the sample, was then positioned in an oven set precisely at 105°C for a duration of six hours. The evaporating dish, now holding the dried sample, was allowed to cool in a desiccator at room temperature. Subsequently, the dish, along with the dried sample, was re-weighed, and this new weight was recorded accurately. The moisture content was calculated using the formula:
% Moisture = (Weight of fresh sample - Weight of dried sample)/(Weight of sample used)×100
Determination of lipid content: Two grams of the powdered sample were placed inside a filter paper, and the paper was subsequently placed into a Soxhlet extractor. This extractor was positioned within a pre-weighed, dried distillation flask. Acetone was introduced into the distillation flask through the condenser attached to the Soxhlet extractor. A cooled water jet was directed into the condenser, facilitating the continuous reflux of the heated solvent. This refluxing action resulted in the extraction of lipids from the sample, accumulating in the solvent chamber. Upon complete extraction of lipids from the sample, the condenser and extractor were disconnected, and the solvent was evaporated to concentrate the lipid extract. The concentrated lipid extract in the flask was then dried in an air oven until a constant weight was achieved. Subsequently, the flask containing the dried lipid extract was re-weighed to accurately measure the weight of the lipid.
The calculation of % Lipid was performed using the formula:
% Lipid = (Weight of flask and extract - Weight of empty flask)/(Weight of sample extracted) ×100
Determination of ash content: One gram of the dried sample was precisely weighed into a preheated and pre-weighed porcelain crucible. The crucible, now containing the sample, was carefully placed into a muffle furnace, where it was exposed to a regulated temperature of 630°C for a period of three hours. Subsequently, the crucible was allowed to cool down to room temperature before being re-weighed. The calculation for % Ash was performed using the formula:
%ASH= ((Weight of crucible + sample) - (Weight of crucible+ sample after ash))/(Weight of sample) ×100
Determination of carbohydrate content: 0.1 g of the sample was precisely weighed and placed into a 25 ml volumetric flask. To this, 1 ml of distilled water and 1.3 ml of 62% perchloric acid were added. The mixture was vigorously shaken for 20 minutes to ensure complete homogenization. The volumetric flask was filled up to the 25 ml mark with distilled water and securely stoppered. The resultant solution was then allowed to settle for decanting purposes. 1 ml was extracted from the filtrate and placed into a 10 ml test tube, followed by dilution to the desired volume with distilled water. Following this, 1 ml of the prepared solution was dispensed into another clean test tube, and 5 ml of Anthrone reagent was introduced. Additionally, a combination of 1 ml distilled water and 5 ml Anthrone reagent was prepared. Both mixtures were read at a wavelength of 630 nm using the prepared blank (1 ml distilled water and 5 ml Anthrone reagent) for calibration purposes. Additionally, a solution of glucose (0.1 ml) was treated as the sample with Anthrone reagent, and its absorbance was measured. The % Carbohydrate as glucose was calculated using the formula:
% CHO as glucose = (25 x absorbance of sample)/(Absorbance of standard glucose)
Determination of crude fiber content: Two grams of the sample underwent extraction using petroleum ether. Subsequently, the sample was refluxed for 30 minutes in the presence of 200 ml of dilute hydrochloric acid, and the resulting mixture was filtered. The obtained residue underwent thorough washing with water until it reached an acid-free state. This residue was then transferred to a beaker and subjected to an additional 30 minutes of boiling, this time with 200 ml of dilute sodium hydroxide solution. Following another filtration, the residue was collected in an ignited crucible. The collected residue underwent a washing process involving three washes with 20 ml ethanol and two washes with 10 ml ether. After the washing steps, the residue was dried in an oven until a constant weight was achieved. The dried residue was then cooled and weighed. Subsequently, the dried residue was placed into a furnace and subjected to ignition. After the ignition process, the residue was cooled once more and weighed. The calculation for crude fiber content involves the following formula:
% Crude Fiber = (Weight of dried residue - Weight of ignited crucible with residue)/(Weight of sample used) ×100
Determination of protein content: 0.1 g of the sample was precisely weighed into a clean 250 ml conical flask. To this, 3 grams of digestion catalyst and 20 ml of concentrated sulphuric acid were added. The mixture was heated to initiate digestion, resulting in a colour change from black to sky-blue. After allowing the digest to cool to room temperature, distilled water was used to dilute it to a total volume of 100 ml. From this diluted digest, 20 ml was measured into a distillation flask. The distillation flask was secured on an electrothermal heater or hot plate and attached to a Liebig condenser, leading to a receiver containing 10 ml of 2% boric acid indicator. Through a syringe attached to the mono-arm steelhead, 40 ml of Sodium hydroxide was incrementally introduced into the digest until it became strongly alkaline. The mixture was then heated to boiling, and the resultant distilled ammonia gas passed through the condenser into the boric acid indicator, causing the colour to change from purple to greenish. The distillate in the receiver was titrated back to a purple colour from the greenish hue using standard 0.1N Hydrochloric acid solution. The volume of hydrochloric acid required for this colour change was noted as the titer value.
The calculation for % Organic Nitrogen (used to estimate protein content) is:
% Organic Nitrogen = (Titre value × Factor × 100 × 100)/(V × W)
Where:
Titer value is the volume of standard acid (e.g., 0.1N Hydrochloric acid) used in titration.
Factor is a conversion factor, often 1.4, representing the conversion of nitrogen to protein.
V is the volume of the sample used in the digestion step (in liters).
W is the weight of the sample used in grams.
Extraction of the plant materials
Using the American National Cancer Institute (NCI) method of extraction, [3], the crude extracts were obtained from 100g of each plant sample which was macerated in organic solventsMethanol and dichloromethane combined in a 1;1 ratio, after which they were concentrated using a rotary evaporator, with the water bath temperature set at 40o C. The extracts were further dried in a desiccator to remove any trace of solvent. The extract was weighed, stored in airtight container and preserved in refrigerator for further analysis.
Partitioning
For the partitioning process, the dichloromethane-methanol extract-also described as the Total extract was utilized. 200 milliliters of n-Hexane were used to dissolve 50 grams of the extract, producing a concentrated solution which could be partitioned. In a separating funnel, the solution was mixed with an equivalent volume of methanol. The components were thoroughly mixed and partitioned by vigorous shaking. After allowing the mixture to settle, two separate layers-a non-polar n-Hexane layer and an aqueous methanol layer-formed. Upon being carefully separated, the n-Hexane layer was set aside. In a separating funnel, the aqueous methanol layer from the first partitioning phase was collected and mixed with an equal volume of n-Hexane. Vigorous shaking and subsequent settling resulted in the separation of two layers: an n-Hexane layer and an aqueous methanol layer. As necessary, these partitioning steps were repeated to maximize the extraction efficiency. To obtain needed compounds, each collected layer was concentrated using a rotary evaporator. Precise recording of the volumes utilized and fraction yield during the partitioning process was archived for analysis and reference.
Phytochemical tests
Preliminary phytochemical screening was carried out on all the crude leaf extracts (dichloromethane and methanol) using standard procedures as described by [4-6].
Antimicrobial susceptibility testing: Agar well diffusion method
Bacteria: The bacterial strains used were Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli. Sterile petri dishes were labelled in triplicate for each bacterial strain. Mueller-Hinton agar pour was uniformly inoculated with 0.1 ml of standardized bacterial cultures and thoroughly mixed. After pouring the inoculated agar into the labelled petri dishes, it was allowed to cool and solidify on the workbench. Then, a sterile cork-borer (6 mm) was used to aseptically create wells in the solidified agar. Preparation of 100% solutions of various extracts (n-Hexane, methanol, and total) was carried out. A few drops of these extract solutions were added into the corresponding wells on the agar plates. The plates were incubated at 37°C for 24 hours under optimal conditions. After incubation, observation for zones of inhibition around each well was conducted, with Gentamicin serving as the positive control and Dimethyl Sulfoxide (DMSO) as the negative control. Accurate measurements of the diameter of these zones were taken using a caliper.
Fungi: The chosen fungal strains were Candida albicans, Fusarium oxysporum, Aspergillus niger, and Penicillium chrysogenum. Sterile petri dishes were labelled in triplicate for each fungal strain. Potato Dextrose agar pour was uniformly inoculated with 0.1ml of standardized fungal cultures and thoroughly mixed. After pouring the inoculated agar into the labelled petri dishes, it was allowed to cool and solidify on the workbench. Then, wells were aseptically created in the solidified agar using a sterile cork borer (6 mm). Preparation of 100% solutions of n-Hexane, methanol, and total extracts was performed. The respective extract solutions were added into the wells on the agar plates. The plates were incubated at 25°C for 72 hours under optimum conditions. Following incubation, observation for zones of inhibition around each well was carried out, using Fluconazole as the positive control and Dimethyl Sulfoxide (DMSO) as the negative control. Accurate measurements of the diameter of these zones were taken with a caliper.
Minimum inhibitory concentration (mic) determination
Sterile petri dishes were labelled in triplicates for each bacterial strain. Mueller-Hinton agar pours were inoculated with 0.1 ml of standardized bacterial cultures, ensuring thorough mixing. The inoculated agar was poured into the labelled petri dishes and left to solidify on the workbench. After solidification of the agar, five (5) discs were carefully extracted from the agar layer using a sterile cork-borer. This process created five (5) wells in each agar plate. These wells were labelled to accommodate five (5) concentrations of each extract identified with activity during the preliminary study. Concentrations included 50 mg/ml, 25 mg/ml, 12.5 mg/ml, 6.25 mg/ml, and 3.125 mg/ml. With a separate sterile Pasteur’s pipette, 0.1 ml of each extract concentration was meticulously added to the corresponding wells. The plates were left on the workbench for 15 minutes to ensure proper diffusion of the extracts. Subsequently, all plates were incubated at 37°C for 24 hours. Post-incubation, the diameter of resulting zones of inhibition were measured in millimeters (mm) using a caliper, directly through the base of the plates.
Antioxidant assay
The DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Assay was employed to evaluate the antioxidant potential of jackfruit (Artocarpus heterophyllus Lam). This assay is widely used. It evaluates how effectively jackfruit compounds can neutralize the stable DPPH radical. As jackfruit extracts interact with DPPH radicals, a colour change occurs from purple to yellow, indicating the scavenging ability. The degree of colour change correlates with the antioxidant potential, with stronger colour changes indicating higher scavenging capacity [7].
Gas chromatography mass-spectroscopy (gc-ms) characterization
Utilizing an Agilent 7890B GC system in conjunction with an Agilent 5977A MSD and a Zebron-5MS column (ZB-5MS 30 m × 0.25 mm × 0.025 μm) (5%-phenylmethylpolysiloxane), the Gas Chromatography Mass Spectrometry (GC-MS) analysis was quantitatively determined. The carrier gas was GC-grade helium, flowing at a steady 2 mL/min rate. Before being used, the total extract was filtered and dissolved in ethanol. An ultimate temperature of 300°C was attained by progressively raising the column temperature from 60°C to 10°C each minute. The GCMS analysis took forty minutes to complete. By using a computer to compare the mass spectra with the NIST 11 MS library (National Institute of Standards and Technology library), the chemicals were identified.
Fourier transform infrared spectroscopy
The FTIR instrument was powered on and allowed to warm up for the specified duration typically 10-15 minutes. The associated computer was turned on, and the FTIR software was launched. A background spectrum was collected without any sample in the instrument’s sample compartment. The prepared sample was carefully placed in the appropriate sample holder within the instrument’s sample compartment. The instrument scanned the sample across a designated wavenumber range, usually 4000-400 cm-1, collecting infrared absorption data. The acquired was displayed on the software’s interface. Relevant spectral features, such as peaks and bands, were identified and analyzed. Spectral manipulation techniques, including baseline correction or peak normalization, were applied as needed to enhance data quality and facilitate interpretation. The collected spectrum was saved in a suitable format for future reference and analysis.
Statistical analysis
Each test was carried out in triplicate. The values were expressed as mean ± Standard Error of Mean (SEM). The Student’s T-test was employed to identify significant distinctions between each column and the control, with a P value < 0.05 considered as statistically significant. All statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA).
Results
Result of physicochemical analysis
Physicochemical analysis was carried out on the fresh pulp of Artocarpus heterophyllus Lam. The moisture content and pH determined is represented in Table 1.
Table 1: Physicochemical properties of the fresh pulp of artocarpus heterophyllus lam.
| Parameter | Value |
|---|---|
| Moisture Content | 77% |
| pH | 7.02 |
Result of proximate analysis
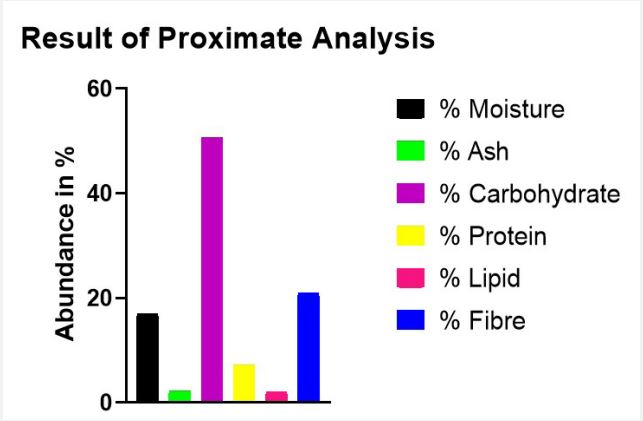
Following the analysis of the dried pulp of Artocarpus heterophyllus Lam. for its Proximate composition, the percentage abundance of the compositions was determined. The Moisture content was determined to be 16.96%; the extract exhibited a lipid content of 2.06%; the Ash content was measured at 2.26%; the fiber content was 20.89%; the protein content was assessed to be 7.20% and the Carbohydrate content was found to be 50.63%. The proximate composition was presented in Figure 1.
Result of phytochemical screening
The Phytochemical screening was carried out on the Total extract of the dried pulp of Artocarpus heterophyllus Lam., the extract exhibited the presence of various bioactive compounds, these Phytochemical constituents mostly responsible for the plant’s biological activities were determined. The phytochemical constituents are presented in Table 2.
Result of dpph antioxidant activity
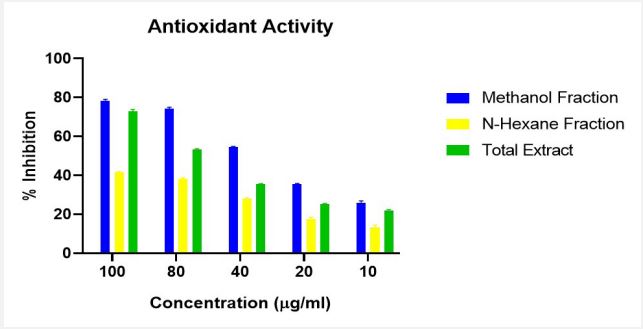
DPPH Assay which utilizes the stable, nitrogen-centered free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) was used to determine the Antioxidant activity of the n-hexane, methanol fractions and total extract of Artocarpus heterophyllus Lam. The percentage inhibition across all concentration is presented in Figure 2. The Half-maximal inhibitory concentration (IC50) values were presented in Table 3.
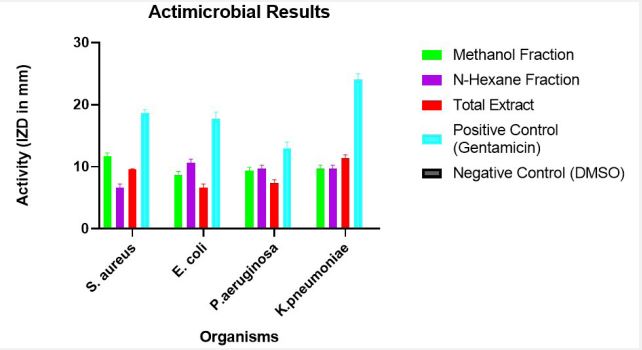
The results presented in Figure 3, showed that methanol fraction demonstrated enhanced antimicrobial activity than the n-hexane fraction and the total extract.
Table 2: Phytochemical constituents of the total extract of Artocarpus heterophyllus Lam.
| Test | Result | Inference |
|---|---|---|
| Alkaloids | Positive | Alkaloids present |
| Tannins | Positive | Tannins presen |
|
Saponins a. Frothing test b. Emulsion test |
Positive Positive |
Saponins present |
| Flavonoids | Positive | Flavonoids present |
|
Carbohydrates a. Fehling’s test b. Molisch’s test |
Positive Positive |
Carbohydrates present |
|
Proteins a. Millions test b. Picric test |
Positive Positive |
Proteins present |
|
Sterols/Triterpenoids a. Liebermann test b. Salkowski test |
Positive Positive |
Triterpenoids present Steroidal nucleus present |
| Phenolics | Positive | Phenolics present |
| Phlobatannins | Positive | Phlobatannins present |
|
Cardiac Glycosides a. Keller Killiani test b. Kedde test |
Negative Negative |
Cardiac Glycosides absent |
Result of antimicrobial susceptibility testing
The n-Hexane, Methanol Fractions and the Total Extract of the dried pulp of Artocarpus heterophyllus Lam. were tested against four pathogenic bacterial species and four pathogenic fungal species, the positive control was Gentamicin for bacterial and Fluconazole for fungi while the negative control was DMSO.
The IC50 values of the extract and fractions were determined and the values presented in Table 3.
Table 3: The Half-maximal Inhibitory Concentration (IC50) val- ues of the n-hexane, methanol fractions and total extract of the dried pulp of Artocarpus heterophyllus Lam.
| Sample | IC50 (μg/ml) |
|---|---|
| Methanol Fraction | 39.19 |
| N-Hexane Fraction | 102.25 |
| Total Extract | 70.10 |
From the result obtained, the n-Hexane, Methanol Fractions and the Total Extract exhibited activity against the panel of bacteria as indicated by the measured inhibitory zone diameter - IZD (in mm) and exhibited no activity against the panel of fungi as indicated by the absence of inhibitory zone diameter - IZD. The Values of IZD obtained are presented in Table 4.
Table 4: Inhibitory Zone Diameter - IZD (in mm) of the n-Hexane, methanol fractions, total extract and controls against pathogenic bacte- rial species.
| Inhibitory Zone Diameter (IZD) | |||||
|---|---|---|---|---|---|
| Organism | Methanol Fraction | N-Hexane Fraction | Total Extract | Positive Control (Gentamicin) | Negative Control (DMSO) |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| Staphylococcus Aureus | 11.667±0.333 | 6.667±0.333 | 9.667±0.333 | 18.667±0.333 | 0.000±0.000 |
| Escherichia Coli | 8.667±0.333 | 10.667±0.333 | 6.667±0.333 | 17.667±0.667 | 0.000±0.000 |
| Pseudomonas Aeruginosa | 9.333±0.333 | 9.667±0.333 | 7.333±0.333 | 13.000±0.577 | 0.000±0.000 |
| Klebsiella Pneumoniae | 9.667±0.333 | 9.667±0.333 | 11.333±0.333 | 24.000±0.577 | 0.0±0.000 |
Key: Results are presented in Mean ± Standard Error of Mean (SEM); Sample Size N=3. The results are further displayed in Figure 3, to obtain an in-depth analysis.
The results presented in Figure 3, showed that methanol fraction demonstrated enhanced antimicrobial activity than the n-hexane fraction and the total extract.
Result of minimum inhibitory concentration
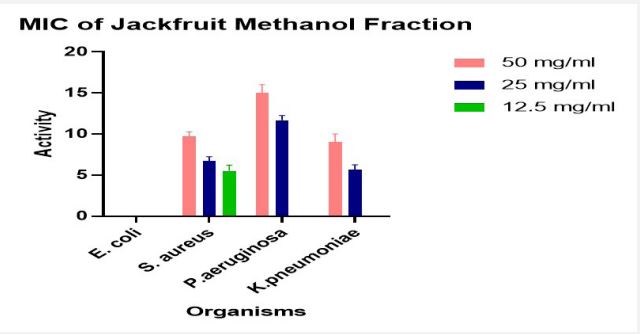
The Minimum Inhibitory Concentration (MIC) of the Methanol Fraction only was presented in Figure 4.
Result of gas chromatography - mass spectroscopy (gc-ms) characterization
The GC-MS Analysis revealed a diverse range of compounds including, Fatty acids (dodecanoic acid, tetra decanoic acid, tridecanoic acid, n-hexadecanoic acid), Fatty acid esters (hexadecenoic acid, methyl ester), Unsaturated fatty acid derivatives (9,12-octadecadienoic acid, methyl ester, 9,12,15-octadecatrienoic acid, methyl ester), Long chain unsaturated hydrocarbon (1-docosene), Chlorinated ester (dichloroacetic acid, 4-hexadecyl ester) and a Carotene derivative (lycopersene). A well detailed GC-MS profile is presented in Table 5.
Result of fourier-transform infrared (ftir) spectroscopy
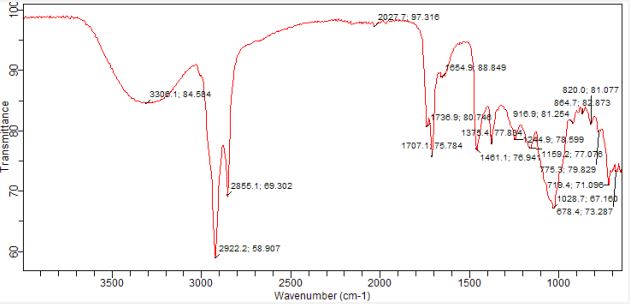
The n-hexane fraction of the dried pulp of Artocarpus heterophyllus Lam. was analysed using FTIR, revealing a diverse array of functional groups and bond types including aliphatic hydrocarbons (alkanes), various oxygenated functional groups (alcohols, esters, aldehydes, ketones, ethers), and aromatic moieties. The bond types and functionalities are presented in Table 6 and Figure 6.
Table 5:Chemical Profile from Gas chromatography - Mass spectroscopy (GC-MS) characterization of the n-hexane fraction of the dried pulp of Artocarpus heterophyllus Lam.
| Peak | Retention Time (MIN) | Area (%) | Molecular Formula | Molecular Weight (g/mol) | Compound | Match Quality(%) |
|---|---|---|---|---|---|---|
| 1 | 20.045 | 0.81 | C12H24O2 | 200.32 | Dodecanoic acid (lauric acid) | 90 |
| 2 | 22.960 | 0.68 | C14H28O2 | 228.37 | Tetradecanoic acid (myristic acid) | 97 |
| 3 | 22.982 | 0.34 | C18H34CL2O2 | 353.40 | Dichloroacetic acid, 4-hexadecyl ester | 91 |
| 4 | 26.644 | 1.46 | C13H26O2 | 214.34 | Tridecanoic acid (tridecylic acid) | 97 |
| 5 | 24.801 | 0.96 | C22H44 | 308.6 | 1-docosene | 90 |
| 11 | 26.866 | 3.92 | C17H34O2 | 270.5 | Hexadecanoic acid, methyl ester | 95 |
| 14 | 29.298 | 6.45 | C16H32O | 256.42 | N-hexadecanoic acid | 99 |
| 15 | 30.800 | 0.45 | C19H34O2 | 294.5 | Methyl linoleate | 97 |
| 19 | 31.339 | 11.05 | C19H32O2 | 292.5 | Methyl linolenate | 98 |
| 22 | 38.38 | 0.28 | C40H66 | 547.0 | Lycopersene | 72 |
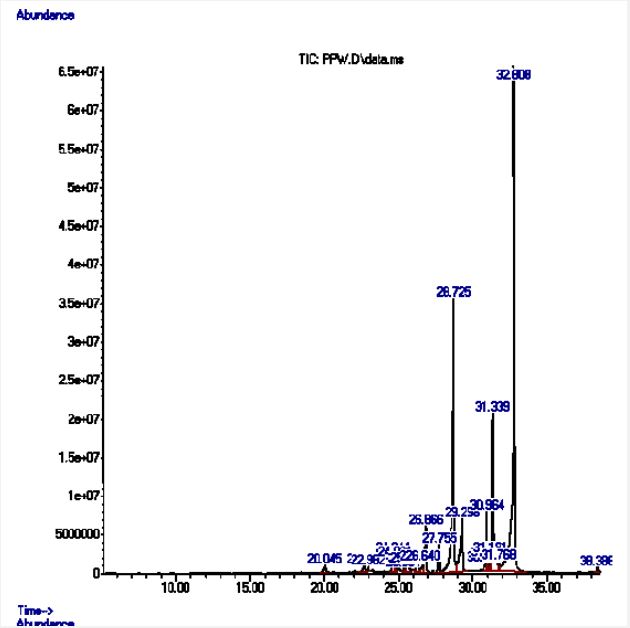
*The highlighted compounds are pharmacologically active. The GC-MS chromatogram is shown in Figure 5, below. A closer look would reveal the presence of 22 compounds, of which 10 were of match quality above 70%.
Table 6: The bond types and functional groups present in dried pulp of Artocarpus heterophyllus Lam.
| (%Wave Number(Cm-1) | Bond Type | Functional Group |
|---|---|---|
| 3306.1 | O-H | Hydroxyl |
| 2922.1 | C-H | Symmetric Methylene (Saturated aliphatic) |
| 2855.1 | C-H | Asymmetric Methylene (Saturated aliphatic) |
| 1738.9 | C=O | Carboxylic, Ester |
| 1707.1 | C=O | Ketone, Aldehyde, Ester |
| 1654.9 | C=O | Alkene |
| 1461.1 | C-H | Methylene |
| 1375.4 | C-H | Symmetric Methyl (Saturated aliphatic) |
| 1244.9 | C-O | Carboxylic, Ester |
| 1159.2 | C-O | Carboxylic, Ether |
| 1028.7 | C-O | Ether |
| 916.9 | C-H | Alkyl substitution |
| 864.7 | C-H | Aromatic C-H substitution |
| 820.0 | C-H | Aromatic C-H substitution |
| 775.3 | C-H | Aromatic C-H substitution |
| 719.4 | C-H | Aromatic C-H substitution |
| 678.4 | C-H | Aromatic C-H substitution |
The FTIR spectrum is shown in Figure 6. The spectrum is rich in aromatic compounds.
The study revealed an average corneal surface temperature of 36°C, exceeding the 34.65°C average reported in the literature [42] or the range of 32.9°C to 36.6°C reported for the average across the cornea (R. Moreddu, M. Elsherif, H. Butt, D. Vigolo, and A.K. Yetisen, Contact lenses for continuous corneal temperature monitoring. This difference may be attributed to variations in boundary conditions and control parameters, such as ambient temperature, blood temperature, and convection coefficient. Additionally, differences in eye geometry modeling across studies could contribute to the observed variance. Figures 5 and 6 compare temperature rises along the eye’s horizontal axis, from the cornea to the sclera. The direct result is that safety procedures should be applied for eye protection in different environments or every day habits [43].
Discussion
The Physicochemical screening reveals the moisture content and pH of the fresh pulp of Artocarpus heterophyllus Lam. as shown in Table 1. Moisture content refers to the presence of water in a material, a critical factor for assessing stability and shelf life. A high moisture content suggests the need for specific preservation methods to prevent degradation and enhance stability. The fresh fruit pulp’s moisture content (77%) closely aligns with findings by [8] in Bangladesh, where jackfruit pulps had moisture contents ranging from 80.95% to 82.22%. Various factors such as climate, soil nature, and fruit maturity can influence the moisture content of a specific variety. While the high moisture content makes jackfruit pulp easily perishable, it also presents opportunities for producing large quantities of juice. Exploring preservation techniques like freeze-drying or processing into food products (jams, syrup, and chips) could optimize storage and availability. The pH level indicates the acidity of a material, with the fresh pulp’s pH of 7.02 slightly higher than that reported by [9] in the Philippines (pH 4.8-5.5). It’s important to note that jackfruit’s pH can vary based on ripeness, growing conditions, and processing. As jackfruit ripens, it becomes less acidic and more alkaline. The fruit’s neutral pH makes it a milder option for sensitive individuals, potentially easing digestion and minimizing gastrointestinal discomfort often associated with acidic fruits.
Proximate analysis serves as a fundamental tool for comprehending the nutritional composition of various materials. It furnishes crucial details about proteins, fats, carbohydrates, fibres, and moisture, aiding in the assessment of essential nutrient and the potential dietary benefits of the product. Fibre, the nondigestible portion of plant-based foods, offers diverse benefits, including stimulating gut movement, promoting smooth digestion, preventing constipation, fostering a healthy gut microbiome, lowering cholesterol levels, and regulating blood sugar. The fibre content obtained (20.89%) from Figure 1. exceeds that reported by [10] which showed that jackfruit pulps in Bangladesh had fibre contents between 1% and 1.5%. This difference in crude fibre from the present study might be due to collection of fruits from different agro-climatic zones indicating the influence of genetic and microclimatic factors. This remarkable crude fibre content positions the pulp of jackfruit as a potent prebiotic source, potentially aiding digestion, regulating blood sugar, and promoting gut health making it valuable for managing conditions like digestive disorders, diabetes and obesity. The extract had a moderate carbohydrate content of 50.63% showing that it can provide readily available energy, making it a valuable dietary source for people with active lifestyles or high energy demands. This carbohydrate content surpasses that reported by [11] in India (31.2%), [12] for dried jackfruit pulp (13.08%), and [13] for jackfruit pulp from Kenya and Uganda (ranging from 21.65% to 24.91%). These differences could be attributed to genetic variations in the jackfruit analysed by the different authors. Jackfruit contains substantial amounts of simple sugars like fructose, glucose and sucrose and is considered a high energy fruit [14]. Additionally, the inherent sweetness of jackfruit, derived from its carbohydrates, provides a healthy substitute for processed sugars and opens up enticing opportunities for crafting delectable and nourishing food items like jams and syrups, as well as chips, snacks, juices, and smoothies. Protein, a vital macronutrient, plays essential roles in the body, contributing to tissue building, repair, and regulation of metabolic processes. The extract revealed a decent protein content of 7.20%. According to [15], it contains amino acids like arginine, cystine, histidine, leucine, lysine, methionine, threonine, and tryptophan. Jackfruit has emerged as a valuable source of plant-based protein for vegetarians, vegans, and anyone seeking to diversify their protein intake. It has a significant protein potential that could be exploited in human nutrition, especially in the production of baby food and livestock feed. That is, Protein fractions could be isolated and incorporated into functional food products or even protein supplements. Moisture content of dried pulp was compared to the fresh pulp of jackfruit (77% moisture), the significantly lower moisture content in the dried pulp (19.69%) inhibits microbial growth and enzymatic activity, leading to a longer shelf life. This allows for easier storage and distribution without rapid spoilage. It also allows for greater flexibility in versatile culinary applications, such as flour alternative, in snacks, cereals, and energy bars. Ash content is the inorganic residue remaining after a sample is completely burned at high temperatures. A higher ash content suggests a potentially richer supply of minerals. The Ash content (2.26%) hints at a rich mineral supply. According to [16], potassium leads the pack; a crucial mineral for acid-base balance, osmotic pressure, and nerve impulses makes jackfruit a valuable dietary source. Calcium, the second most abundant mineral, is vital for bones, blood clotting, and nerve transmission, further encouraging its consumption, especially for growing children and those with osteoporosis. Phosphorus, another bone and teeth strengthener, further contributes to jackfruit’s health benefits, particularly for children and nursing mothers. While the 2.06% lipid content is low, its fatty acid profile and potential health benefits deserve further investigation.
Phytochemical screening (Table 2) demonstrated a diverse array of bioactive constituents in the pulp of Artocarpus heterophyllus, hinting at its potential for antioxidant and antimicrobial activity. This aligns with studies by [17-20], who identified various phytochemicals like carotenoids, flavonoids, tannins, and sterols in jackfruit pulp, albeit in varying concentrations depending on the variety. Flavonoids, phenolics, and tannins boast powerful antioxidant properties, scavenging free radicals and protecting against oxidative damage. Certain tannins and phlobatannins further contribute by chelating metal ions involved in free radical generation. Sterols/terpenoids offer additional antioxidant and anti-inflammatory potential, enriching the extract’s overall antioxidant profile and suggesting potential for combating oxidative stress and associated chronic diseases. The extract also holds promise for antimicrobial applications. Tannins possess broad-spectrum activity against bacteria and fungi, while some saponins and phenolics disrupt bacterial membranes, and specific terpenoids target bacterial membranes or inhibit biofilm formation. These diverse phytochemicals pave the way for potential development of antimicrobial treatments.
The DPPH assay, which utilizes the stable free radical DPPH to measure antioxidant activity, revealed a dose-dependent increase in scavenging abilities for all samples, well presented in Figure 2. The Methanol fraction consistently exhibited the strongest activity across all concentrations (78% inhibition at 100 μg/ml), indicating the presence of polar antioxidants preferentially extracted by methanol. This aligns with the study of [21] which found that methanol extracts often show the highest antioxidant activity, correlated with phenolic and flavonoid contents. While the n-Hexane fraction possessed the weakest activity (41%-13% inhibition), it still displayed measurable scavenging capacity, suggesting the presence of non-polar antioxidants with lower potencies; the low activity across all concentrations could imply limited abundance or solvent solubility of these compounds. The Total Extract occupied a middle ground (72%- 21% inhibition), suggesting a diverse mix of antioxidants with varying potencies, likely including some beyond those found in the methanol fraction.
Half-maximal inhibitory concentration, IC50, as shown in Table 3, which quantifies the amount of a substance needed to achieve 50% inhibition of free radical activity, further confirmed the Methanol Fraction’s dominance with a “Very strong” activity (IC50=39.191 μg/ml). The n-Hexane Fraction displayed “medium” activity (IC50=102.25 μg/ml), while the Total Extract exhibited “strong” activity (IC50=70.10 μg/ml). This classification of the strength of Antioxidant activity is based on the study of [22]. Overall, the evidence strongly suggests that the Methanol Fraction harbours the most potent antioxidants, providing a promising avenue for further exploration and potential applications.
The Antimicrobial analysis was carried out and the result presented in Table 4 and Figure 3. The panel of bacteria used are Gram positive - Staphylococcus aureus and Gram negative - Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae. Interestingly, the n-Hexane fraction showed comparatively better activity against the gram-negative bacteria. This could likely be because this fraction is non-polar and would have an easier access via the Lipopolysaccharide outer membrane of gram-negative bacteria, compared to the Methanol fraction which is polar. Notably, though they act as broad spectrum, showing activity against both gram positive and gramnegative bacteria, there could also be development of the fractions to show activity targeted at a type of bacteria.
The Minimum Inhibitory Concentration (MIC) is a measure of the lowest concentration of an antimicrobial agent that completely prevents the visible growth of a microorganism. As presented in Figure 4. the MIC obtained was 12.5 mg/ml for Staphylococcus aureus and 25 mg/ml for both Pseudomonas aeruginosa and Klebsiella pneumoniae. This shows that the Methanol fraction is more potent against Staphylococcus aureus than the other organisms.
GC-MS analysis of the n-Hexane fraction revealed 22 major peaks, of which 10 were selected based on match quality and similarity to library compounds as shown in Table 5. Peak area is the reflection of the amount of specific analyte that is present. This is based on the number of counts taken by the mass spectrometer detector at the point of retention. Therefore, the peak area is proportional to the amount of the component.
Fatty acids comprised a significant portion of the detected compounds, including lauric acid, myristic acid, tridecylic acid, palmitic acid, and methyl esters of hexadecanoic, linoleic, and linolenic acids. Notably, methyl linolenate exhibited the highest peak area (11.05%), indicating its substantial presence. Other notable compounds included 1-docosene, a long-chain unsaturated hydrocarbon, and a low-abundance lycopersene, a modified carotene derivative. Saturated fatty acids (lauric, myristic, tridecylic) while generally possessing weak antimicrobial activity, can disrupt bacterial membranes, leading to cell death. Palmitic acid, with a high peak area of 6.45%, is a notable exception, exhibiting moderate antimicrobial activity against various bacteria, primarily Gram-positive strains, as supported by [23]. Methyl linoleate demonstrated moderate antimicrobial activity by disrupting microbial membranes and biofilms, as well as moderate antioxidant activity due to its two double bonds that scavenge free radicals. Methyl linolenate, with its three double bonds, exhibited strong antimicrobial and antioxidant properties, likely playing a significant role in the sample’s overall activity, as indicated by its high peak area (11.05%). Lycopersene, a Deca hydro-carotenoid, likely exhibits weak antimicrobial activity due to reduced membrane interaction from hydrogenation, but still possesses strong antioxidant activity due to its double bonds’ ability to scavenge free radicals and reactive oxygen species [24,25].
The FTIR spectrum (Figure 6) and interpretation of spectrum (Table 6) paints a detailed picture of the n-hexane fraction’s complex chemistry. Strong peaks at 2922.2 and 2855.1 cm-1, along with a peak at 1375.4 cm-1, reveal aliphatic hydrocarbons in the form of alkanes with methylene (CH2 ) and Methyl (CH3 ) groups. Additionally, a peak at 1654.9 cm-1 suggests the presence of alkenes. Beyond hydrocarbons, the broad peak around 3306.1 cm-1 indicates hydroxyl, while peaks at 1707.1 and 1738.9 cm-1 point to ketones, esters, or aldehydes. Further evidence of oxygenated functional groups comes from peaks at 1244.9 and 1159.2 cm-1 (esters, carboxylic acids, or ethers) and a distinct peak at 1028.7 cm-1 (ethers). The spectrum also reveals multiple peaks between 864.7 and 719.4 cm-1 suggesting substituted aromatic compounds. Comparing with the GC-MS and Phytochemical screening result, the Strong aliphatic hydrocarbon peaks & methyl group peak could be due to the presence of fatty acids. Alkene peak validates the double bond of 1-docosene. Carbonyl stretching peaks and further C-O stretching evidence support the presence of the methyl esters of fatty acids. Hydroxyl peak could be due to flavonoids and phenolics present.
Conclusion
This study comprehensively explores the bioactivity of the pulp of Artocarpus heterophyllus Lam., revealing its remarkable potential as a natural source of both antioxidants and antimicrobials. This study reveals that the pulp of Artocarpus heterophyllus Lam. possesses significant antioxidant activity, with the Methanol Fraction showcasing the highest efficacy and potency compared to the Total Extract and n-Hexane Fraction. This suggests the presence of potent antioxidant compounds preferentially extracted by methanol. The order of Antioxidant activity is Methanol fraction > Total Extract > n-Hexane Fraction. The study also reveals that the pulp of Artocarpus heterophyllus Lam. possesses significant antimicrobial activity, particularly against bacteria. All fractions, including Methanol, n-Hexane, and the Total Extract, showed significant activity against the tested bacterial panel. Interestingly, the Methanol Fraction displayed superior activity against Gram-positive bacteria, while the n-Hexane Fraction proved more effective against Gram-negative bacteria.
References
- CDC, Center for Disease Control and Prevention. (2022). The biggest antibiotic-resistant threats in the U.S. https://www.cdc.gov/drugresistance/biggest-threats.
- Ranasinghe, R. A. S. N., Maduwanthi, S. D. T., & Marapana, R. A. U. J. (2019). Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllusLam.): A Review. International Journal of Food Science, 2019, 1-12.
- Thomas, G. (2010): High Throughput Extraction of Plant, Marine and Fungal Specimens for Preservation of Biologically Active Molecules. Molecules. 15, 4526
- Trease, G. E., and Evans, W. C. (2002): Pharmacognosy. Saunders Publishers
- Sofowora, A. (1993): Recent trends in research into African medicinal plants. Journal of ethnopharmacology, 38(2-3), 209-214.
- Harborne, J. B. (1998): Phytochemical methods: a guide to modern techniques of plant analysis. Springer Science & Business Media.
- Loizzo, M., Tundis, R., Chandrika, U., Abeysekera, A., Menichini, F., & Frega, N. (2010). Antioxidant and Antibacterial Activities on Foodborne Pathogens of Artocarpus heterophyllus Lam. (Moraceae) Leaves Extracts. Journal of Food Science, no-no.
- Goswami, C., & Chacrabati, R. (2016). Chapter 14-jackfruit (Artocarpus heterophyllus). In M. S. J. Simmonds & V. R. Preedy (Eds.), Nutritional composition of fruit cultivars (Vol. 2016, pp. 317-335).
- Galvez, L., & Dizon, E. (2017). Physico-chemical and Functional Properties of Two Jackfruit (Artocarpus heterophyllus Lam.) Varieties in Eastern Visayas, Philippines. Annals of Tropical Research, 100-106.
- Haq, N. (2006). Jackfruit (Artocarpus heterophyllus). In J. T. Williams, R. W. Smith, & Z. Dunsiger (Eds.), Tropical fruit trees (pp. 107-132). Southampton Centre for Underutilised Crops, University of Southampton, UK
- Chrips, N. R., Balasingh, G. R., & Kingston, C. (2008). Nutrient constituents of neglected varieties of Artocarpus heterophyllus Lam. From Kanyakumari district, South India. Journal of Basic and Applied Biology, 2(3&4), 36-37.
- Shafiq, M., Mehmood, S., Yasmin, A., Khan, S. J., Khan, N. H., & Ali, S. (2017). Evaluation of Phytochemical, Nutritional and Antioxidant Activity of Indigenously Grown Jackfruit (Artocarpus heterophyllus Lam). Journal of Scientific Research, 9(1), 135-143.
- Ojwang, R., Muge, E., Mbatia, B., Mwanza, B., & Ogoyi, D. (2017). Comparative Analysis of Phytochemical Composition and Antioxidant Activities of Methanolic Extracts of Leaves, Roots and Bark of Jackfruit (Artocapus heterophyllus) from Selected Regions in Kenya and Uganda. Journal of Advances in Biology & Biotechnology, 16(1), 1-13.
- Chowdhury, F. A., Azizur Raman, M., & Jabbar Mian, A. (1997). Distribution of free sugars and fatty acids in jackfruit (Artocarpus heterophyllus). Food Chemistry, 60(1), 25-28.
- Pavanasasivam, G., Uvais, M., & Sultanbawa, S. (1973). Cycloartenyl acetate, cycloartenol and cycloartenone in the bark of Artocarpus species. Phytochemistry, 12(11), 2725-2726.
- Kamdem Bemmo, U. L., Bindzi, J. M., Tayou Kamseu, P. R., Houketchang Ndomou, S. C., Tene Tambo, S., & Ngoufack Zambou, F. (2023). Physicochemical properties, nutritional value, and antioxidant potential of jackfruit (Artocarpus heterophyllus) pulp and seeds from Cameroon eastern forests. Food Science & Nutrition, 11(8), 4722-4734.
- Arung, E., Shimizu, K., & Kondo, R. (2007). Structure-Activity Relationship of Prenyl‐Substituted Polyphenols from Artocarpus heterophyllus as Inhibitors of Melanin Biosynthesis in Cultured Melanoma Cells. Chemistry & Biodiversity, 4(9), 2166-2171.
- Chandrika, U., Jansz, E., & Warnasuriya, N. (2004). Analysis of carotenoids in ripe jackfruit (Artocarpus heterophyllus) kernel and study of their bioconversion in rats. Journal of the Science of Food and Agriculture, 85(2), 186-190.
- Ong, B., Nazimah, S., Osman, A., Quek, S., Voon, Y., Hashim, D. M., Chew, P., & Kong, Y. (2006). Chemical and flavour changes in jackfruit (Artocarpus heterophyllus Lam.) cultivar J3 during ripening. Postharvest Biology and Technology, 40(3), 279-286.
- Wong, K. C., Lim, C. L., & Wong, L. L. (1992). Volatile Flavour Constituents of Chempedak (Artocarpus polyphema Pers.) Fruit and Jackfruit (Artocarpus heterophyllus Lam.) from Malaysia. Flavour and Fragrance Journal, 7(6), 307-310.
- Sreeja Devi, P. S., Kumar, N. S., & Sabu, K. K. (2021). Phytochemical profiling and antioxidant activities of different parts of Artocarpus heterophyllus Lam. (Moraceae): A review on current status of knowledge. Future Journal of Pharmaceutical Sciences, 7(1).
- Farhamzah, F., Kusumawati, A. H., Alkandahri, M. Y., Hidayah, H., Sujana, D., Gunarti, N. S., Yuniarsih, N., Apriana, S. D., & Agustina, L. S. (2022). Sun Protection Factor Activity of Black Glutinous Rice Emulgel Extract (Oryza sativa var glutinosa). Indian Journal of Pharmaceutical Education and Research, 56(1), 32-310.
- Wang, W., Wang, R., Zhang, G., Chen, F., & Xu, B. (2020). In Vitro Antibacterial Activities and Mechanisms of Action of Fatty Acid Monoglycerides Against Four Foodborne Bacteria. Journal of Food Protection, 83(2), 331-337.
- Kusmita, L., Nur Prasetyo Edi, A., Dwi Franyoto, Y., Mutmainah, Haryanti, S., & Dwi Retno Nurcahyanti, A. (2023). Sun protection and antibacterial activities of carotenoids from the soft coral Sinularia sp. symbiotic bacteria from Panjang Island, North Java Sea. Saudi Pharmaceutical Journal, 31(8), 101680.
- Fiedor, J., & Burda, K. (2014). Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients, 6(2), 466-488.