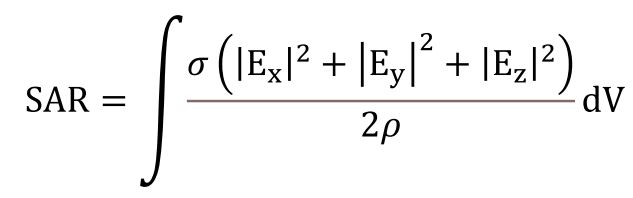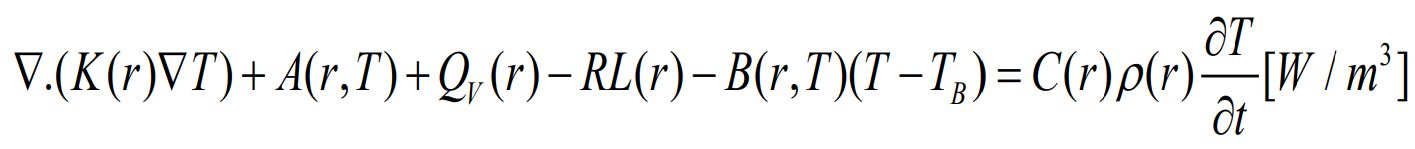Research Article
Volume 3, Issue 4
Digital Twins for Risk Prediction of Microwave Radiation in the Human Eye
Adamopoulou Maria1 ; Makrynioti Dimitra1 ; Fouras Athanasios1 ; Messaris Gerasimos1 ; Hatzilygeroudis Ioannis2 ; Koutsojannis Constantinos1*
1Department of Physiotherapy (Incl Optics & Optometry Department), Laboratory of Health Physics & Computational Intelligence, School of Rehabilitation Sciences, University of Patras, Patras, Greece.
2Computer Engineering & Informatics Department, AI Group, University of Patras, Patras, Greece.
Corresponding Author :
Koutsojannis Constantinos
Email: ckoutsog@upatras.gr & madamo@upatras.gr
Received : Mar 08, 2024 Accepted : Apr 26, 2024 Published : Apr 30, 2024 Archived : www.meddiscoveries.org
Citation: Adamopoulou M, Makrynioti D, Fouras A, Messaris G, Hatzilygeroudis I, Koutsojannis C. Digital Twins for Risk Prediction of Microwave Radiation in the Human Eye. Med Discoveries. 2024; 3(4): 1147.
Copyright: © 2024 Constantinos K. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Recent studies support the long-held belief that the lens of the eye is one of the human body’s most radiosensitive tissues, especially in the ocular region. This study highlights the influence of non-ionizing electromagnetic radiation, which may cause cataracts or other visual illnesses. We exposed intact cultured lenses to microwave radiation for extended periods while maintaining a consistent ambient temperature using an A.I. computer-controlled electromagnetic bench. To simulate using a cell phone, both eyes exposed to microwave radiation at 0.8 to 1.2 GHz and 2.0 W from a distance of 2 to 5 cm. The investigation of Electromagnetic Field (EMF) distribution in living tissues simulated using the Sim4Life platform’s MIDAS head phantom for personalized experiments through “Digital Twin” approaches. According to our research, this electromagnetic radiation causes a mean rapid up to 2°C temperature increase. Notably, electromagnetic radiation causes temperature-related effects on the sclera, choroid, and retina, which are the posterior portions of the eye. This explained by the predominant heat flux that these regions’ blood flow produces. Extended exposure (i.e., more than 20 minutes) to 2.0 W at 1.1 GHz is detrimental to the eyes’ health and optical performance. The temperature elevation is the main cause of possible damaging processes for the nonionizing radiation band, according to International Regulation Bodies such as ICRP and ICNIRP. The study unequivocally shows that microwave radiation has an impact on the eye structures, introducing risks with both immediate and long-term consequences. The optical transmission properties of these structures compromised by electromagnetic radiation that surpasses specific energy thresholds, indicating potential harm to them. The current methodology can applied to personalize workplace/environment safety measures or even medication techniques for eye tissues, as all Digital Twins models.
Keywords: Eye; Personalized care; Digital twins; Microwave radiation.
Introduction
The eye is a truly remarkable and complex organ, distinguished from other organs in various ways. Notably, the cornea and lens, vital for vision, remain clear and devoid of blood vessels throughout a person’s life, unlike other non-vascular tissues that we regularly lose and regenerate, such as hair, nails, and teeth [1]. Another unique feature is the retina, which develops early during fetal growth as an extension of the brain, preserving many brain-like features. The retinal vascular system is the only one visible directly in a living body, making the eye a valuable tool for doctors to observe and diagnose various health conditions like hypertension, diabetes, sickle-cell anemia, and age-related changes [1]. Measuring about 25 millimeters across, the eye is almost a perfect sphere, comprising the cornea, aqueous chamber, iris, lens, and vitreous body. The cornea, approximately 12 millimeters wide, is crucial for focusing light, while the lens adjusts for near and far vision, especially in younger individuals [2]. The iris regulates light entering the eye by altering pupil size. Filled with aqueous humor and vitreous body, primarily water and a gel-like substance, the spaces between these components maintain the eye’s structural integrity. The retina, a layered, light-sensitive area, consists of nerve cells and photoreceptors, supported by the choroid and retinal blood vessels. The sclera, a tough, white layer, provides external protection. Clear and properly shaped components, along with a fully functional retina, are essential for optimal vision, converting light into signals for the brain [1]. Cataracts induced by ionizing radiation, such as X-rays and gamma rays, typically appear in the posterior part of the lens, often as posterior subcapsular cataracts. Increased exposure to ionizing radiation leads to greater opacity in the lens, with this effect occurring over a shorter latency period [3]. The formation of cataracts due to ionizing radiation is associated with damage to the lens cell membrane, akin to cataracts caused by microwaves. Another potential mechanism involves damage to the DNA of lens cells, resulting in alterations in protein concentrations, reduced synthesis of protective enzymes, and decreased formation of sulfur-sulfur bonds [4,5]. Until conclusive findings on the causes of microwave- and ionizing radiation-induced cataracts are established, and alternative preventive strategies identified, the recommendation is to consider mechanical shielding against these radiations [3]. Studies have explored how external factors like electromagnetic radiation, infrared rays, and sunlight influences the eye’s temperature profile [5-7]. For instance, research has investigated how wearing glasses can modify ocular temperature in different weather conditions and how ocular temperature changes with age. Models assessing the impact of radiofrequency radiation have demonstrated an increase in ocular temperature due to external thermal effects. The adverse effects of prolonged exposure to infrared radiation have also been examined [8- 10]. Microwaves influence living tissue through thermal and nonthermal mechanisms. Experimental animals have shown induced lens opacities at relatively high intensities, specifically power densities exceeding 100 mW/cm2 . Lens changes at lower intensities may depend on the cumulative dose. At “nonthermal intensities,” microwaves can act as triggers, initiating changes in living tissues, such as Ca++ efflux. Some agents known to cause cataracts, such as alloxan and galactose, exhibit synergistic effects with microwaves. Microwaves have also observed to expedite cataract formation in individuals with diabetes. Microwaves, either alone or combined with certain drugs, can damage the corneal endothelium. Animals exposed to microwaves have shown degeneration of retinal nerve endings and a slight increase in retinal permeability. However, the impact of long-term, low-intensity microwave exposure on the human lens remains inadequately understood. Several reports have linked occupational microwave exposure to an increased incidence of lens aging and retinal injury among microwave workers. In Canada, the recommended microwave exposure limits are set at 25 mW/cm2 for microwave workers and at 1 mW/cm2 for the general public, both averaged over 1 minute. The Australian microwave, exposure safety standard from 1985 suggests pre- and post-employment eye examinations for workers in this field. The reality of potential exposure of significant portions of the population to intricate, multifrequency microwave radiation in the environment necessitates the establishment of a safe exposure level for the public. This is essential to prevent any occurrence of adverse effects without unnecessarily restricting the beneficial applications of microwaves. Biological effects resulting from exposure to microwave radiation commonly categorized as either thermal or non-thermal (specific) in nature. Thermal effects arise from the heating of biological specimens and can be replicated using traditional heating methods. On the other hand, non-thermal or specific effects result from the direct interaction between the electromagnetic field of incoming microwave radiation and the biological specimen. The testicles and eyes are particularly sensitive to temperature elevation, making them the most vulnerable organs to microwave radiation exposure. Studies on dogs, rabbits, and rats have revealed that at 10 mW/cm2 (power density in milliwatts per square centimeter), pathological damage to the testes includes degeneration of the epithelium lining of the seminiferous tubules and a significant reduction in the number of maturing spermatocytes. The reduction in testicular function due to the heating effect at 10 mW/cm2 seems to be temporary and reversible. Cataracts have induced in the eyes of experimental animals, with threshold values for cataract formation established in rabbits at approximately 100 mW/cm2 for long-term exposure to Continuous Wave (CW) radiation. Reports of human eye cataracts due to microwave exposure have documented at power densities of a similar magnitude. However, further research needed to specify threshold values for cataract formation in humans. Detecting non-thermal or specific effects is more challenging than thermal effects due to the nature of the biological specimen’s response and the lack of a clear explanation for the mechanism causing the effect. Low-level microwave radiation has been associated with neurological effects in animals, including changes in EEG patterns, conditioned reflexes, sensitivity to stimuli, alterations in cerebral cortex biocurrents, and behavioral changes. Human workers around microwave equipment have reported subjective symptoms, as documented by researchers from the U.S.S.R. and Eastern Europe. Genetic effects have reported, with exposure to microwave radiation inducing abnormal development in chick embryos, while conventional heating to the same temperature did not produce abnormalities. These abnormalities appear to link to the inhibition of growth and cell differentiation caused by a direct interaction with the electromagnetic field, rather than thermal effects.
Related work
There is a close relationship between higher temperature, body or ambient, and problems in the anterior-posterior ocular area, even if the cornea demonstrates a regulatory system of plateauing the corneal temperature to up to 36o C regardless of a continuous increase in the ambient or body temperatures [11]. Bellow there is a summary of possible temperature-related eye diseases and injuries.
Tear film: Beginning from the outermost layer, tear film is highly affected by higher environmental temperatures. Higher temperatures can cause ocular dryness, tear film instability and increased osmolality, ocular discomfort or even inflammation, and consequently, visual disturbance.
Crystalline lens: As regards a deeper organ, the crystalline lens, it has implicated that there is a close correlation between increased eye temperature and lens problems, such as cataract and presbyopia [11]. Additionally, the relationship between UV radiation and cataract formation is well established [12-15]. As it has early established in animal models though [16], environmental temperature has also a great impact in cataract formation, as it rises the body [11] and crystalline lens temperature [17-19]. This is typical in both sun-associated [19] and othersources associated [20] increase in ambient temperature. Tropical and subtropical countries show higher occurrence of nuclear cataract formations [21]. What is more, a most recent study revealed that temperatures as low as 10oF (=5.5oC) higher in some countries can result in 44% more cases of severe vision impairment in older adults [22]. This association between ambient temperature and cataract development may be constant but not fully understood, as many factors seem to play a role into this association [23-25].
Retina: What is more, higher temperature is correlated with higher incidence of retinal detachment. A timely population study of 11 years showed higher ambient temperatures to be one of the two main reasons for the occurrence of retinal detachment [26]. Temperature and seasonality are also closely correlated with special forms of retinal detachment, such as rhegmatogenous [27-29] and tractional [30] retinal detachment (Pterygium, Pinguecula).
Other ocular formations that can lead to sight loss, such as pterygium and pinguecula, are highly correlated with UV light and environmental factors such as high ambient temperatures and dry eye [31].
Inflammation: Ocular inflammation is affected by climate. Trachoma, which, according to World Health Organization, is “a disease of the eye caused by infection with the bacterium Chlamydia trachomatis”, is a serious a public health problem present in 42 countries, responsible for the visual impairment or even irreversible blindness of about 1.9 million people [32]. Main risk factor for Chlamydia trachomatis to be attached, live and infect the eye is higher temperatures, accompanied with inadequate hygiene and crowded population in poorer rural areas of Africa, Central and South America, Middle East, Asia, Australia.
Microbial keratitis: Another vision-threatening condition closely related to higher temperatures is microbial keratitis, and more specific fungal keratitis [33].
Lastly, we address the limitations of existing diagnostic methods, such as retinal photography and fluorescein angiography, in detecting retinal damage from low-level blue or violet light exposure. These methods fall short in explaining the underlying mechanisms of such changes. To bridge this gap, we’re developing new techniques that leverage the fluorescent properties of probe dyes, offering potential insights into the mechanisms behind retinal damage. This research is not just about diagnosing damage but also understanding its root causes to improve preventive and treatment strategies. Interest in the temperature profiling of the human eye, using computer simulations and numerical methods, is growing among researchers using Digital twins moving to personalized medicine [34]. In both laboratory animals and humans, as evidenced by epidemiological studies and documented cases, microwaves are frequently implicated in the occurrence of anterior and/or posterior subcapsular lenticular opacities, commonly referred to as cataracts. There appears to be a direct correlation between the intensity of the microwaves and the duration of exposure with the development of cataracts [4]. Heat-sensitive enzymes, such as glutathione peroxide, which typically protect lens cell proteins and membrane lipids from oxidative damage, undergo distortion as part of the cataract formation process. The oxidation of protein sulfhydryl groups and the formation of high-molecular-weight aggregates induce local variations in the ordered structure of lens cells.
A number of studies have proven the relation between different types of non-ionizing radiation and eye health. In his 1991 study, Okuno investigated the thermal effects of Infrared Radiation (IR) on the human eye, focusing on occupational settings with high IR exposure, like those involving molten glass or steel. The research developed a theoretical model to simulate IR exposure in the human eye, calculating temperature distributions within. Key findings indicated that occupational cataracts may arise from IR absorption in the cornea and heat conduction to the lens. Okuno established threshold IR irradiances for cataract formation, which are between 163-178 mW/cm2 for longterm exposure under normal conditions. This threshold could be halved for workers performing heavy work in high ambient temperatures. This study was instrumental in providing guidelines for IR exposure limits in workplaces to prevent cataract formation.
In their 2013 study, [35]. Investigated the impact of laser radiation on the human eye, emphasizing the organ’s vulnerability to heat sources, particularly in laser-based medical procedures. Utilizing finite element method simulations, the research examined how different lasers, including the 1064 nm Nd: YAG, 193 nm ArF excimer, and 1340 nm Nd: YAP, affect the eye’s retina, lens, and cornea. The results revealed a significant temperature rise in these areas, highlighting the potential for tissue damage. This study was crucial for understanding the risks in laser eye surgeries and now presents a non-invasive method to evaluate and reduce these risks.
Study [36] has studied the impact of non-ionizing electromagnetic radiation on the bovine eye lens, using a controlled experimental setup. Bovine lenses were incubated in an organ culture system for 15 days, during which they were exposed to 1.1GHz microwave radiation. The findings have indicated that exposure exceeding 36 hours impairs the lens’s optical functionality, although temporary recovery was observed if the exposure is halted. Microscopic analysis revealed bubbles at the lens sutures, suggesting a damage mechanism distinct from a temperature increase. At the end of the experiment, persistent radiation damage is noted at the lens sutures, along with a decrease in lens epithelial enzyme activities. This research shaded light on both the immediate and prolonged effects of electromagnetic radiation on the eye lens, emphasizing the importance of understanding and addressing these impacts in environments with electromagnetic exposure.
Additionally to the previous, a number of computer simulation approaches are using theoretical models to run experiments in computer environment. In their 2022 study, Md. Ashiqur Rahman and Mamun Rabbani explored the effects of LED light exposure on human eye temperature. The researchers conducted simulations using a novel COMSOL Multiphysics 5.3 to model the temperature profile of the human eye when exposed to Red, Green, and Blue LED light. The results indicated that Blue LED light caused the most significant temperature rise on the corneal surface, up to 0.91°C, compared to rises of 0.85°C and 0.46°C for Green and Red LED lights, respectively. However, the posterior layers of the eye, including the sclera, choroid, and retina, experienced minimal thermal effects due to the dominant heat flux from blood flow in those regions. This study offered valuable insights into the thermal impact of LED light exposure on the eye, particularly relevant in the context of the increasing use of LED lighting in various devices and artificial environments. As a common framework, this approach is lately described as the patient’s DIGITAL TWIN because of the imaging-based adaptive detailed anatomical models of different human body parts under investigation [37].
Material and methods
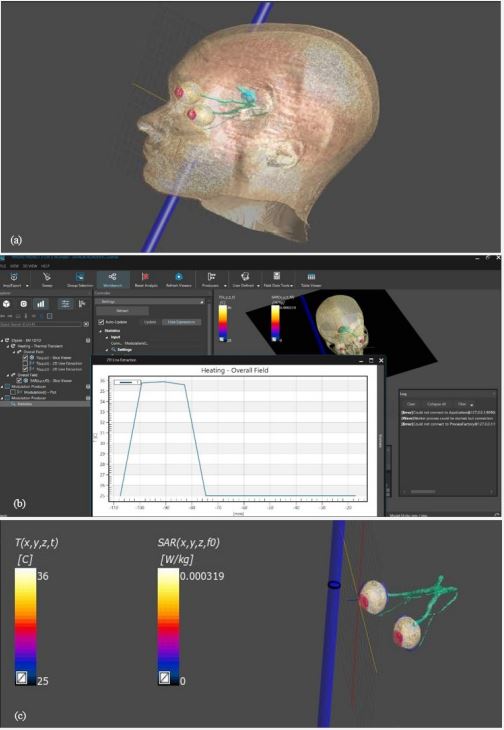
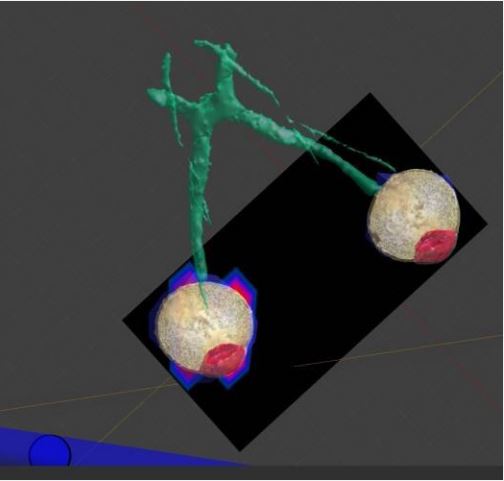
To The present study focuses on evaluating the impact of non-ionizing electromagnetic radiation on eye tissue, specifically by monitoring the rise in eye temperature across various wavelengths. A digital model employed to simulate eye tissue, integrating optical physics for non-ionizing electromagnetic radiation and bioheat transfer physics to assess thermal effects [38]. The simulation and analysis utilized a sophisticated program based on the “MIDA” model, a multimodal imaging-based detailed anatomical model of the human head and neck developed at the Institute for Biomedical Engineering (ETH Zurich, Switzerland) [39]. The MIDA model, constructed using scans from a 3 Tesla MRI scanner, incorporates multiple MRI modalities to enhance signals of specific tissues, particularly the eye and ear structures. High-resolution anatomical images obtained from these MRIs provide a comprehensive understanding of the intricate anatomy of the eye, crucial for accurate simulations. The model integrates sequences such as a 3D MagnetizationPrepared Gradient Echo Sequence (MPRAGE) and a 3D Turbo Spin Echo (TSE) sequence, both offering 500μm isotropic resolution for superior imaging of eye and ear regions. Vascular imaging achieved using Magnetic Resonance Angiography (MRA) scans, essential for understanding the vasculature of the head, including the eye.
This study investigates the impact of non-ionizing electromagnetic radiation exposure on eye tissue by monitoring the rise in eye temperature caused by specific electromagnetic wavelengths. Simulations considered the microstructure of the brain to understand the radiation impact on adjacent ocular tissues. Diffusion-weighted imaging sequences provided insights into tissue anisotropy and fiber orientation. Datasets from various sources co-registered for precise alignment and integration, facilitating an accurate representation of the human eye in simulations. The segmentation process, performed using iSeg software, allowed for the precise delineation of different ocular structures. This advanced computational modeling enabled the simulation of the thermal effects of non-ionizing electromagnetic radiation on the human eye with unprecedented accuracy, significantly contributing to the understanding of its potential consequences. The model includes homogenous sections: cornea, aqueous fluid, lens, iris, ciliary body, vitreous humor, retina, choroid, sclera, and optic nerve, as presented in Figure 1. The model’s eye dimensions include a mean 24 mm pupillary axis and a mean 22.5 mm vertical axis diameter and can personalized with the use of imaging inputs.
SAR distribution
There are international guidelines in place to protect humans from the potentially harmful effects of Radiofrequency Electromagnetic Fields (RF-EMF). Institutions such as the International Commission on Non-Ionizing Radiation Protection [40] and the Institute of Electrical and Electronics Engineers (IEEE) specify these guidelines. The limits set by these institutions, known as “basic restrictions,” are derived using the metric of the average Specific Absorption Rate (SAR) for a part of the human body or the entire body (Equation 1).
· E: RMS value of electric field in tissue (V/m)
· σ:Tissue conductivity (S/m)
· ρ: Tissue density (kg/m3)
SAR measurement procedures are commonly applied to mobile phones within microwave band. Understanding electromagnetic pollution and SAR is essential for conducting thorough risk assessments, establishing safety guidelines, guiding technological advancements, and promoting public awareness regarding the potential health effects of RF-EMF exposure. Although numerous recent studies have shown that ambient RFEMF levels are generally below established limits, it is crucial to carefully determine the doses absorbed by the human body or parts of it, to fully evaluate the potential health implications for each individual because of different workplace and living environments [41]. Continued research in this field is vital to ensure that individuals can make informed decisions about their RFEMF exposure and adopt measures to minimize potential risks. Here we present our approach for human eyes.
Temperature distribution
The temperature profile of the human eye, unaffected by external factors, deduced from biological processes within the eye. This profile generated by solving Pennes’ bio heat transfer equation, detailed in Equation 2 [2]:
· ρ represents the density of the tissue, measured in kilograms per cubic meter.
· C symbolizes the specific heat capacity, expressed in Joules per kilogram per Kelvin.
· ω denotes the rate of blood flowing through the tissue, measured in reciprocal seconds.
. k represents the thermal conductivity of the tissue, quantified in Watts per meter per Kelvin, indicating how well the tissue can conduct heat.
· Q is the rate of heat generation within a given volume of tissue, stemming from the body’s metabolic processes or external sources.
· T is the temperature, measured in Kelvin.
· t is time, in seconds.
Table 1: Thermal properties of the homogenous ocular regions.
| Ocular Region |
Thermal Conductivity k (Wm-'K-1) |
Specific Heat C (JKg-'K-1) |
Density P (Kgm-3) |
|---|---|---|---|
| Cornea | 0.58 | 4178 | 1050 |
| Aqueous Humor | 0.58 | 3997 | 996 |
| Lens | 0.4 | 3000 | 1050 |
| Iris | 1.0042 | 3180 | 1100 |
| Vitreous | 0.603 | 4178 | 1000 |
| Sclera | 1.0042 | 3180 | 1100 |
| Retina | 0.565 | 3680 | 1039 |
| Choroid | 0.530 | 3840 | 1040 |
| Ciliary | 0.498 | 3340 | 1040 |
* modified from [37].
In this complex interplay of variables, blood plays a pivotal role, indicated by the subscript b. The temperature gradient within the eye model is expressed by ∇ (k∇T). Blood flow’s impact on temperature is modeled in the equation as ωρbcb (t - tb). Q, where Tb, is the blood temperature. The term Q at the end of the equation encapsulates both internal and external sources of heat. This comprehensive equation captures the intricate dynamics of heat transfer and generation within the eye tissues [37].
In the context of this study, what is noteworthy is the relatively minor role of blood flow in influencing temperature changes, especially when compared to external heating sources. This realization led also other researchers to exclude the blood flow term, ωρbcb (T-Tb), from their simulation of heat transfer in the eye. This decision helps to simplify the model without significantly influencing its accuracy.
The specific thermal properties that were used in the simulation are meticulously listed in Table 1 of the study, providing a detailed reference for those interested in the nitty- gritty of the methodology [2]. This table likely includes values for parameters such as tissue density (ρ), specific heat capacity (c), thermal conductivity (k), blood perfusion rate (ω), and others, offering a comprehensive overview of the thermal properties considered in the simulation.
Heat transfer simulation
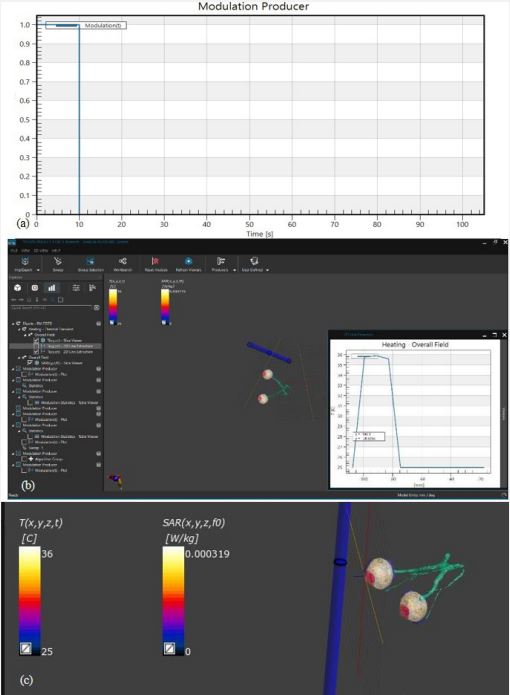
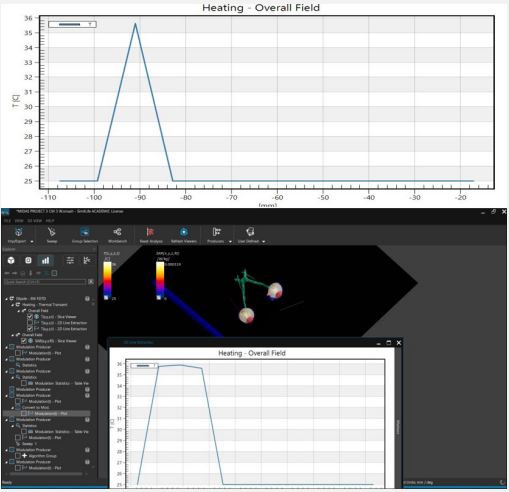
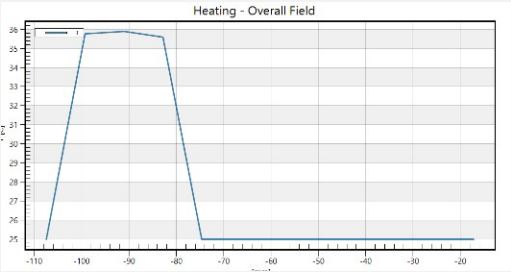
In our investigation into the thermal dynamics of the eye, particularly under the influence of non-ionizing electromagnetic radiation, we focused on analyzing three-dimensional temperature landscapes at key points within the eye. These critical points include the very front of the cornea, the front and back tips of the lens. The selection of these locations based on the observation that electromagnetic radiation tends to have a more pronounced impact on temperatures in the cornea, the aqueous humor, and the lens compared to other parts of the eye. Our findings are visually depicted in Figures 3 to 4, where temperature profiles at these strategic eye locations are mapped out.
MIDA employed
Results
To provide a point of reference, we incorporated a baseline temperature curve that illustrates the thermal state of the eye in the absence of electromagnetic radiation exposure. This curve is presented as a solid line and is referred to as the ‘normal conditions’ scenario.
The study revealed an average corneal surface temperature of 36°C, exceeding the 34.65°C average reported in the literature [42] or the range of 32.9°C to 36.6°C reported for the average across the cornea (R. Moreddu, M. Elsherif, H. Butt, D. Vigolo, and A.K. Yetisen, Contact lenses for continuous corneal temperature monitoring. This difference may be attributed to variations in boundary conditions and control parameters, such as ambient temperature, blood temperature, and convection coefficient. Additionally, differences in eye geometry modeling across studies could contribute to the observed variance. Figures 5 and 6 compare temperature rises along the eye’s horizontal axis, from the cornea to the sclera. The direct result is that safety procedures should be applied for eye protection in different environments or every day habits [43].
Discussion
In our article delves into the impact of nonionizing radiation on eye tissues and explores ways to identify and understand the morphological changes or injuries caused by such radiation exposure [44]. We commence by examining how the cornea, especially its front cellular layer, affected by infrared radiation from carbon dioxide lasers [45]. In this context, we employ an innovative model to predict thermal damage, establishing a connection between harm and a phase transition phenomenon. This model explains why temperature increases at the damage threshold vary minimally with different exposure times. Additionally, we explore the healing responses post-exposure [46]. The subsequent segment of our study shifts focus to the cornea’s back cellular layer, analyzing alterations caused by low-level microwave radiation. Histological studies indicate a link between these changes and cell death. These observations have prompted APL to establish more stringent microwave exposure safety limits than those currently recommended by federal guidelines [47]. According to common meeting of ICRP and IDNIRP: “Ionizing radiation can cause stochastic and deterministic effects, while most effects due to exposure from non-ionizing radiation appear to be deterministic. For ionizing radiation, there is a greater emphasis on optimization of protection even at low levels of exposure, whereas for non-ionizing radiation there is a greater emphasis on keeping exposures below thresholds (Reference Levels) for observed effects” [40]. Reference levels have determined based on temperature elevation risk more than 10 C in tissue units, organs or even in all body. The purpose of this study was to determine how the human eye’s temperature changes in response to non-ionizing electromagnetic radiation [38]. The investigation looked at how heat distribution occurs inside the eye because of energy absorption by intraocular tissues from non-ionizing or electromagnetic radiation. Crucial to this process are the cooling of the cornea and the heating of the sclera. In summary, the study’s important findings emphasized as follows:
According to published research, the baseline temperature of an unexposed human eye is about °C higher than normal at the corneal surface.
The absorption of light energy causes the ocular media to become hotter when exposed to non-ionizing electromagnetic radiation.
The deeper layers of the eye, including the choroid, retina, and sclera, have temperatures that are about 2°C higher than average for brief periods of time. This is mostly because blood flow in these tissues has a cooling impact.
Because of temperature elevation, the onset risk of many above-mentioned diseases or accidents increased when the human eye receives microwave radiation inside the microwave band.
Personalized risk assessments are achievable through the application of DIGITAL TWIN methodologies.
Safety procedures should developed to minimize the interaction of EMF radiation with human body or specific body parts [48].
The parameters of a healthy eye, which represent a person’s temperature profile, served as the basis for this investigation. Research on the connection between the temperatures of healthy and sick eyes is still ongoing. The results of this investigation may prove useful in the diagnosis of ocular disorders associated with anomalous temperature profiles. Hopping that future improvement to this study will increase its applicability to real-world scenarios involving the calculation of work-related health hazards [38].
References
- Atchison DA, Smith G. (2000). Optics of the human eye. Encyclopedia of Modern Optics. 2000; 5: 43-63.
- Ng EYK, Tan JH, Acharya UR, Suri JS. (Eds.). Human eye imaging and modeling. 2012.
- Kleiman NJ, Edmondson EF, Weil MM, Fallgren CM, King A, et al. Radiation cataract in Heterogeneous Stock mice after γ-ray or HZE ion exposure. Life Sci Space Res (Amst). 2024; 40: 97-105. doi: 10.1016/j.lssr.2023.09.004.
- Ainsbury EA, Bouffler SD, Dörr W, Graw J, Muirhead CR, et al. Radiation cataract genesis: A review of recent studies. Radiat Res. 2009; 172(1): 1-9.
- Hirata A. Improved heat transfer modeling of the eye for electromagnetic wave exposures. IEEE transactions on biomedical engineering. 2007; 54(5): 959-961.
- Buccella C, De Santis V, Feliziani M. Prediction of temperature increase in human eyes due to RF sources. IEEE Transactions on Electromagnetic Compatibility. 2007; 49(4): 825-833.
- Hirata A. Temperature increase in human eyes due to near-field and far-field exposures at 900 MHz, 1.5 GHz, and 1.9 GHz. IEEE Transactions on Electromagnetic Compatibility. 2005; 47(1): 68-76.
- Bhandari A, Bansal A, Sinha N. Effect of aging on heat transfer, fluid flow and drug transport in anterior human eye: A computational study. Journal of Controlled Release. 2020; 328: 286-303.
- Samaras T. Thermal modeling of the ageing eye. Image Analysis and Modeling in Ophthalmology. 2014; 337-54.
- Scott JA. The computation of temperature rises in the human eye induced by infrared radiation. Physics in Medicine & Biology. 1988; 33(2): 243.
- Kessel L, Johnson L, Arvidsson H, Larsen M. The relationship between body and ambient temperature and corneal temperature. Invest Ophthalmol Vis Sci. 2010; 51: 6593-7.
- Ivanov, I. V., Mappes, T., Schaupp, P., Lappe, C., & Wahl, S. (2018). Ultraviolet radiation oxidative stress affects eye health. J Biophotonics, 11, e201700377.
- Hampel, U., Elflein, H. M., Kakkassery, V., Heindl, L. M., & Schuster, A. K. (2022). [Alterations of the anterior segment of the eye caused by exposure to UV radiation]. Ophthalmologe, 119, 234-239.
- Hatsusaka, N., Yamamoto, N., Miyashita, H., Shibuya, E., Mita, N., Yamazaki, M., Shibata, T., Ishida, H., Ukai, Y., Kubo, E., Cheng, H. M., & Sasaki, H. (2021). Association among pterygium, cataracts, and cumulative ocular ultraviolet exposure: A cross-sectional study in Han people in China and Taiwan. PLoS One, 16, e0253093.
- Tenkate T, Adam B, Al-Rifai RH, Chou BR, Gobba F, et al. WHO/ILO work-related burden of disease and injury: Protocol for systematic reviews of occupational exposure to solar ultraviolet radiation and of the effect of occupational exposure to solar ultraviolet radiation on cataract. Environ Int. 2019; 125: 542-553.
- Kramár P, Harris C, Guy AW. Thermal cataract formation in rabbits. Bio electromagnetics. 1987; 8: 397-406.
- Sliney DH. Physical factors in cataractogenesis: Ambient ultraviolet radiation and temperature. Invest Ophthalmol Vis Sci. 1986; 27: 781-90.
- Kojima, M., Okuno, T., Miyakoshi, M., Sasaki, K., & Takahashi, N. (2002). Environmental temperature and cataract progression in experimental rat cataract models. Dev Ophthalmol, 35, 125-34.
- Al-Ghadyan A, Cotlier E. Rise in lens temperature on exposure to sunlight or high ambient temperature. Br J Ophthalmol. 1986; 70: 421-6.
- Sharon, N., Bar-Yoseph, P. Z., Bormusov, E., & Dovrat, A. (2008). Simulation of heat exposure and damage to the eye lens in a neighborhood bakery. Exp Eye Res, 87, 49-55.
- Sasaki, H., Jonasson, F., Shui, Y. B., Kojima, M., Ono, M., Katoh, N., Cheng, H. M., Takahashi, N., & Sasaki, K. (2002). High prevalence of nuclear cataract in the population of tropical and subtropical areas. Dev Ophthalmol, 35, 60-9.
- Fuller-Thomson, E., Deng, Z., & Fuller-Thomson, E. G. (2023). Association Between Area Temperature and Severe Vision Impairment in a Nationally Representative Sample of Older Americans. Ophthalmic Epidemiol, 1-8.
- Alves, M., Asbell, P., Dogru, M., Giannaccare, G., Grau, A., Gregory, D., Kim, D. H., Marini,
- M. C., Ngo, W., Nowinska, A., Saldanha, I. J., Villani, E., Wakamatsu, T. H., Yu, M., & Stapleton, F. (2023). TFOS Lifestyle Report: Impact of environmental conditions on the ocular surface. Ocul Surf, 29, 1-52.
- Craig, J. P., Alves, M., Wolffsohn, J. S., Downie, L. E., Efron, N., Galor, A., Gomes, J. A. P., Jones, L., Markoulli, M., Stapleton, F., Starr, C. E., Sullivan, A. G., Willcox, M. D. P., & Sullivan, D. A. (2023). TFOS Lifestyle Report Executive Summary: A Lifestyle Epidemic - Ocular Surface Disease. Ocul Surf, 30, 240-253.
- Johnson, G. J. (2004). The environment and the eye. Eye (Lond), 18, 1235-50.
- Lin, H. C., Chen, C. S., Keller, J. J., Ho, J. D., Lin, C. C., & Hu, C. C. (2011). Seasonality of retinal detachment incidence and its associations with climate: an 11-year nationwide population-based study. Chronobiol Int, 28, 942-8.
- Bertelmann, T., Cronauer, M., Stoffelns, B., & Sekundo, W. (2011). [Seasonal variation in the occurrence of rhegmatogenous retinal detachment at the beginning of the 21st century. Study results and literature review]. Ophthalmologe, 108, 1155-63.
- Kim, D. Y., Hwang, H., Kim, J. H., Moon, B. G., Hyung, S. M., Kim, J. Y., & Chae, J. B. (2020). The Association between the Frequency of Rhegmatogenous Retinal Detachment and Atmospheric Temperature. J Ophthalmol, 2020, 2103743.
- Prabhu, P. B., & Raju, K. V. (2016). Seasonal Variation in the Occurrence of Rhegmatogenous Retinal Detachment. Asia Pac J Ophthalmol (Phila), 5, 122-6.
- Auger, N., Rhéaume, M. A., Bilodeau-Bertrand, M., Tang, T., & Kosatsky, T. (2017). Climate and the eye: Case-crossover analysis of retinal detachment after exposure to ambient heat. Environ Res, 157, 103-109.
- Akbari, M. (2022). Update on overview of pterygium and its surgical management. J Popul Ther Clin Pharmacol, 29, e30-e45.
- Solomon, A. W., Burton, M. J., Gower, E. W., Harding-Esch, E. M., Oldenburg, C. E., Taylor,
- H. R., & Traoré, L. (2022). Trachoma. Nat Rev Dis Primers, 8, 32.
- Labeille-Poizat, É., Cornut, P. L., Poli, M., Feldman, A., De Bats, M., Sebilleau, V., Cheggour, M., Denis, P., & Burillon, C. (2013). [Clinical and microbiological features of severe infectious keratitis during heatwaves]. J Fr Ophtalmol, 36, 732-9.
- Kamel Boulos, M. N., & Zhang, P. (2021). Digital twins: from personalised medicine to precision public health. Journal of Personalized Medicine, 11(8), 745.
- Mirnezami, S. A., Jafarabadi, M. R., & Abrishami, M. (2013). Temperature distribution simulation of the human eye exposed to laser radiation. Journal of Lasers in Medical Sciences, 4(4), 175.
- Dovrat, A., Berenson, R., Bormusov, E., Lahav, A., Lustman, T., Sharon, N., & Schachter, L. (2004). Effects of Non–Ionizing Electromagnetic Radiation on the Eye Lens. Investigative Ophthalmology & Visual Science, 45(13), 386-386.
- Rahman, M. A., & Rabbani, M. (2022). Simulation Based Temperature Profiling of Human Eye due to the Exposure of LED Light. Dhaka University Journal of Science, 70(1), 34-41.
- Adamopoulou M, Makrynioti D, Fouras A, Koutsojannis C (2024) Human Radiation-Induced Eye Diseases: A Scoping Review towards “In-silico” Experimental Studies. Ophthalmol Res Rep 8: 159.
- Iacono, M. I., Neufeld, E., Akinnagbe, E., Bower, K., Wolf, J., Vogiatzis Oikonomidis, I., & Angelone, L. M. (2015). MIDA: a multimodal imaging-based detailed anatomical model of the human head and neck. PloS one, 10(4), e0124126.
- ICNIRP (2020) Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys 118(00):000–000; 2020.
- Andrikopoulos, A., Adamopoulos, A., Seimenis, I., Koutsojannis, C. (2017). Microwave diathermy in physiotherapy units: A survey on spatial and time heterogeneity of the electromagnetic field. Journal of Radiological Protection, 37(2), N27-N41.
- Ng, E. Y. K., & Ooi, E. H. (2006). FEM simulation of the eye structure with bioheat analysis. Computer Methods and Programs in Biomedicine, 82(3), 268-276.
- Koutsojannis, C., Andrikopoulos, A., Adamopoulos, A., Seimenis, I. (2018). Microwave diathermy in physiotherapy: Introduction and evaluation of a quality control procedure. Radiation Protection Dosimetry, 181(3), 229-239.
- Jacobson BS. (2005). Cataracts in retired actinide-exposed radiation workers. Radiat Prot Dosimetry, 113(1), 123-5. doi: 10.1093/rpd/nch427. Epub 2004 Nov 23. PMID: 15561739.
- Okuno, T. (1991). Thermal effect of infra-red radiation on the eye: a study based on a model. The Annals of Occupational Hygiene, 35(1), 1-12.
- Blakely E, Kleiman NJ, Neriishi K, Chodick G, Chylack LT, Cucinotta FA, et al. (2010). Radiation Cataractogenesis: Epidemiology and biology. Radiat Res, 173, 709–17.
- ICRP (2007) Recommendations of the International Commission on radiological protection. ICRP publication 103. Ann ICRP 37: 1-332.
- Koutsojannis, C.M. (2018). Electromagnetic radiation from medical equipment: The need for safety procedures due to the time heterogeneity of field strength. Electromagnetic Radiation: History, Theory and Research, 117-34.