Short Commentary
Volume 3, Issue 4
Typical Electrocardiographic Features in Arrhythmogenic Left Ventricular Cardiomyopathy
Stefan Peters*
Internal Medicine and Cardiology, UEK Norden, Germany.
Corresponding Author :
Stefan Peters
Tel: +49-4931-181-435;
Email: h.u.s.Peters@t-online.de
Received : Mar 02, 2024 Accepted : Apr 08, 2024 Published : Apr 15, 2024 Archived : www.meddiscoveries.org
Citation: Peters S. Typical Electrocardiographic Features in Arrhythmogenic Left Ventricular Cardiomyopathy. Med Discoveries. 2024; 3(4): 1142.
Copyright: © 2024 Peters S. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Short commentary
Since 2020 arrhythmogenic cardiomyopathy includes a right dominant form, a biventricular form and a left dominant form.
Already in 2004 Carvajal syndrome was first described in Ecuador within families [1]. This syndrome includes a slighty dilated, poor contracting left ventricle and hair and skin abnormalities with curly hair and plantopalmar keratosis. The genetic background were mutations of the desmoplakin gene.
Primarily it was thought to be a special form of dilated cardiomyopathy. But in ongoing years, with the help of cardiac MRI findings with severe fibrosis, the similarities with Naxos disease with similar aspects of hair and skin were more and more evident.
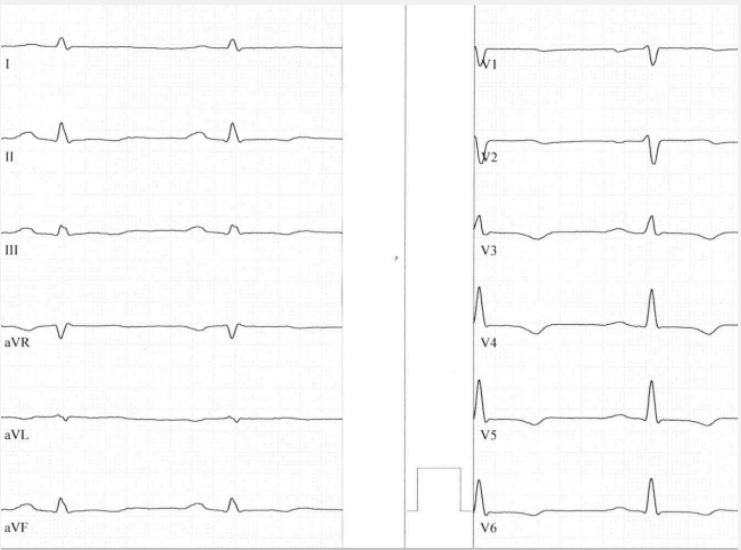
In 2020 Domenico Corrado characterized the term of arrhythmogenic left ventricular cardiomyopathy with extensive fibrosis of the left ventricle and ring-like appearance in cardiac MRI [2]. In many cases low voltage in limb leads and T-wave inversions in inferolateral leads was the characteristic findings in electrocardiography. Figure 1 revealed a typical example of these ECG changes due to a mutation in phospholamban.
Low voltage in limb leads is due to the fact that the left ventricle is poor contracting, T-wave inversions in inferolateral leads are often seen in biventricular or left dominant forms characterizing poor left ventricular function.
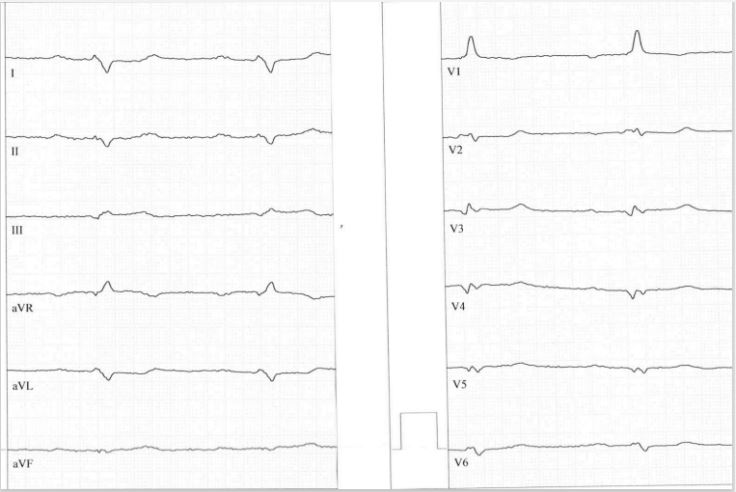
Another typical form of ECG in arrhythmogenic left ventricular cardiomyopathy is described by Calo [3,4] and is characterized by low voltage in limb leads, a very rare left posterior fascicular block both characterizing decrease in LV function, Q waves as electroanatomic scar and dominant shaped R wave in lead V1. The last feature is probably due to mutations of desmoglein-2, desmocollin-2 or in rare cases mutations in plakoglobin (Naxos disease) resembling right ventricular hypertrophy cause by myocyte cell necrosis [5].
Figure 2 revealed a typical example of these ECG changes.
In conclusion, most ECG’s reveal low voltage in limb leads and T-wave inversions or flattening in inferolateral leads. This is especially true in desmoplakin [6], filamin C [7] phospholamban mutations [8], and TMEM43 [9] mutations causing arrhythmogenic left dominant cardiomyopathy with right bundle branch block – like ventricular tachycardia [10].
The second typical ECG of arrhythmogenic left ventricular cardiomyopathy is very rare form and is associated with desmoglein-2 [11] and desmocollin-2 mutations [12]. A positive R/S ratio in lead V1 characterize right ventricular hypertrophy due to right ventricular myocyte cell necrosis [5]. Left posterior fascicular block in combination with low voltage in limb leads characterizes poor left ventricular function. In comparison to desmoglein-2 positive right dominant arrhythmogenic cardiomyopathy typical right precordial epsilon waves and T-wave inversions are missing [13,14].
These two electrocardiographic features characterize arrhythmogenic left ventricular cardiomyopathy besides cardiac imaging especially cardiac MRI. The value of 12-lead ECG in arrhythmogenic left ventricular cardiomyopathy is outstanding.
References
- Kaplan SR, Gard JJ, Carvajal-Huerta L, Ruiz-Cabezos JC, Thiene G, et al. Strutural and molecular pathology oft he heart in Carvajal syndrome. Cardiovasc Pathol. 2004; 13: 26-32.
- Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Ciprinani A, et al. Diagnosis od arrhythmogenic cardiomyopathy: The Padua criteria. Int J Cardiol. 2020; 319: 106-114.
- Calo L, Oliviero G, Crescenzi C, Romeo F, Matino A, et al. Electrocardiogram in arrhythmogenic cardiomyopathy. 2023.
- Calo L, Crescenzi C, Matino A, Casella M, Romeo F, et al. The diagnostic value of the 12-lead ECG in arrhythmogenic left ventricular cardiomyopathy: Novel ECG signs. JACC Electrophysiol. 2023; 9: 2615-2627.
- Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, et al. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med. 2009; 206: 1787-802.
- Rampazzo A, Nava A, Malacrid S, Beffagna G, Bauce B, et al. Mutation in human desmoplakin domain binding to plakoglobin causes dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002; 71: 1200-1206.
- Hespe S, Isbister JC, Duflou J, Puranik R, Bagnall RD, et al. A case series of patients with filamin C truncatring variants attending a specialized cardiac genetic clinic. Eur Heart J Case Rep. 2023; 7: ytad572.
- Vafiadaki E, Glijnis P, Doevendans PA, Kranias EG, Sanoudou D. Phospholamban R14del disease: The past, the present and the future. Front. Cardiovasc. Med. 2023; 10: 1162205.
- Matos J, Helle E, Care M, Moayedi Y, Gollob MH, et al. Cardiac MRI ans clinical outcomes in TMEM43 arrhythmogenic cardiomyopathy. Radiol Cardiovasc Imaging. 2023; 5: e230155.
- Belhassen B, Laredo M, Roudijk RW, Peretto G, Zahavi G, et al. The prevalence of left and right bundle branch block morphology ventricular tachycardia amongst patients with arrhythmogenic cardiomyopathy and sustained ventricular tachycardia: Insights from the European Survey of arrhythmogenic cardiomyopathy. Europace. 2022; 24: 285-295.
- Peters S. Electrocardiographic signs of necrosis and calcification in arrhythmogenic cardiomyopathy. Ann Cardiol Vasc Med. 2023; 6: 1074.
- Brodehl A, Belke DD, Garnett L, Martens K, Abdelfatah N, et al. Transgenetic mice overexpressing Desmocollin-2 (DSC2) develop cardiomyopathy associated with myocardial inflammation and fibrotic remodeling. PLoS One. 2017; 12: e0174019.
- Hermida A, Fressart V, Hidden-Lucet F, Donal E, Probst V, et al. High risk of heart failure associated with desmoglein-2 mutations compared to plakophilin-2 mutations in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Heart Fail. 2019; 21: 792- 800.
- Peters S, Wittinger T. Electrocardiographic signs of right ventricular hypertrophy in desmoglein-2 and plakophilin-2 related arrhythmogenic cardiomyopathy. SL Clin Exp Cardiol. 2020; 3: 120.