Review Article
Volume 3, Issue 4
Trends in the Application of Stem Cells for the Treatment of Diabetes
Jember Tesfahun Bekele*; Gizaw Solomon Tebeje
Department of Medical Biochemistry, College of Health Science, Addis Ababa University, Ethiopia.
Corresponding Author :
Jember Tesfahun Bekele
Email: tesfahunbekele@yahoo.com
Received : Mar 01, 2024 Accepted : Apr 04, 2024 Published : Apr 11, 2024 Archived : www.meddiscoveries.org
Citation: Bekele JT, Tebeje GS. Trends in the Application of Stem Cells for the Treatment of Diabetes. Med Discoveries. 2024; 3(4): 1141.
Copyright: © 2024 Bekele JT. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Diabetes mellitus is a global epidemic that showed a dramatic worldwide increase in its incidence and has been associated with life threatening complications if not detected and managed properly. Even though, insulin and oral anti-diabetic drugs were used for its management, lots of efforts are made to improve patient’s care by pancreas and islet transplantation along with stem cell therapies like embryonic stem cells, induced pluripotent stem cells and adult mesenchymal stem cells. Adult stem cells, which are also termed as somatic stem cells or resident stem cells, are a type of undifferentiated cells, resided in a differentiated tissue, organ or organism, in a specialized structural local microenvironment, called stem cell niche. The stem cell niche, is the in vivo microenvironment where stem cells are located and receive extrinsic signals interact and integrate to influence stem cell behavior that generally determine their fate. ESC showed unlimited differentiation into insulin producing β-cells with ethical concerns. MSCs possess the capacity to differentiate into islet-like insulin producing cells, to promote the regeneration of pancreatic islet beta cells and protect endogenous pancreatic islet beta cells from apoptosis through immunomodulatory mechanisms.
Keywords: Diabetes; Stem cells; Treatment; Insulin.
Introduction
Diabetes Mellitus (DM) is a group of chronic metabolic disorders, which is non-communicable disease characterized by increased blood glucose (hyperglycemia) that leads to the development of severe life-threatening complications. The condition hyperglycemia is due to insufficient insulin secretion by pancreas or inefficient insulin action. The term “diabetes” Greek for siphon which mean ‘melting down of flesh and limbs in to urine’ is introduced by Aretaeus of Cappadocia in the firstcentury CE [1]. In 1675 the name “diabetes mellitus” was coined by Dr. Thomas Willis of England by adding Mellitus which mean honey in Latin due to the sweet taste of urine, contributed for the naming of the disease [2].
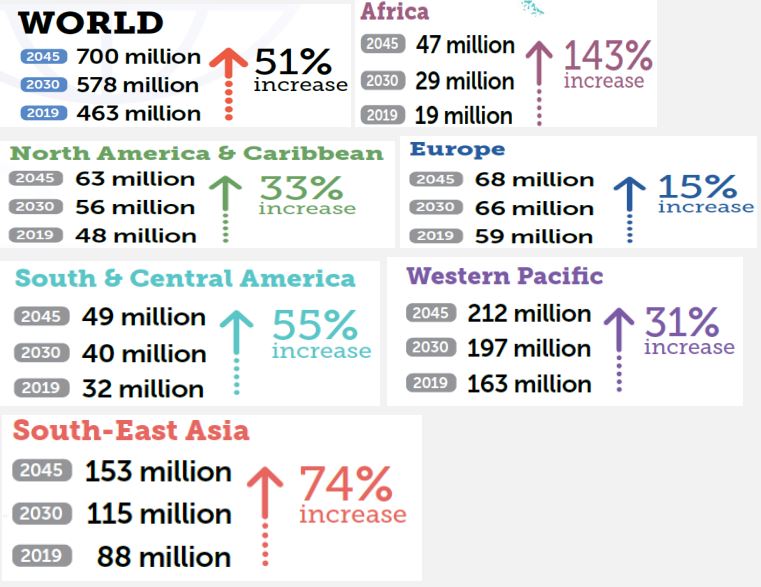
Diabetes is one of the largest global public health problems among others in the world of all nations of developing and developed countries. It imposes a serious global burden on public health as well as socio-economic development. Although its incidence has shown a decrease in some countries, in recent decades the prevalence of diabetes has increased in most developed and developing countries. The highest age-standardized mortality and morbidity with diabetes was observed in Oceania, Sub-Saharan Africa, Southeast Asia, Central Latin America regions and Caribbean countries. The lowest age-standardized mortality and morbidity rates were observed in High Income regions like that of Central Europe, Eastern Europe and East Asia regions. The disease burden in these regions was either remaining stable or showing a slight decrease [3]. In the year 2017 the International Diabetes Federation (IDF) have estimated that 451 million adults were living with diabetes worldwide. This number has increased to 463 million in the year 2019 which is expected to rise to 578 million in 2030 and projected to increase to 700 million in 2045 if effective prevention methods are not adopted Figure 1 below 4 [3,4]. Studies on diabetes prevalence among children and adolescents have showed an increase. From these studies the estimates of children and adolescents below 20 with type 1 diabetes exceed one million. High blood glucose is one of the top 10 causes of death in the world and causes 4 million deaths each year with annual health care cost of US $ 850 billion in 2017 [5]. Over 80% of premature non-communicable deaths are accounted by diabetes together with cardiovascular disease, cancer and respiratory disease [6]. Being diabetics is associated with increased mortality and morbidity from infections, cardiovascular disease, stroke, chronic kidney disease, chronic liver disease and cancer. Although progress has been made in promoting population health and extending life expectancy, diabetes is the second negative effect on reducing life expectancy worldwide [6,7]. In recent years, there was significant increase in the global burden of diabetes and expected to increase in the coming few decades (Figure 1). The effects of diabetes have been extended beyond the individual and affect their families and whole societies, with broad socio-economic consequences and threaten national productivity and economies [5,7].
Variations in the Geographical distribution of diabetic burden with highest prevalence was observed in countries like, China (89.5 million), India (67.8 million), United States (30.7 million), Indonesia (21.0 million) and Mexico (13.1 million) respectively. Highest deaths due to diabetes have been observed in five countries like, India (254,555), China (153,185), Indonesia (97,005), United States (68,558) and Mexico (64,067) [3].
Criteria for the diagnosis of diabetes
Clinically diabetes is characterized by a continuous increase of blood glucose concentration. For the diagnosis of diabetes, measurement of blood glucose level at different situations has been used. For its diagnosis the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus recommends the following criteria. One approach is replicate fasting blood glucose level that is greater than 126 mg/dl (>7 mmol/L) with or without symptoms. Fasting blood glucose levels of 100 mg/ dl or above are considered Impaired Fasting Glucose (IFG). Persons with IFG levels (FPG=100-125 mg/dl (5.6-6.9 mmol/l) and/ or with Impaired Glucose Tolerance Test (IGT) (2 hour post-load glucose 140-199 mg/dl (7.8 mmol/L-11.1 mmol/L) are at risk of developing diabetes and should be observed periodically to detect hyperglycemic progression. After two-hour glucose tolerance test >200 mg/dl (>11.1 mmol/L) is an indicator for being diabetes [9]. A random blood glucose level of ≥11.1 mmol/L (200 mg/dl) with signs and symptoms of diabetes is also an important indicator in the diagnosis of diabetes. In addition to these persons with glycated hemoglobin (HbA1C) value ≥6.5% (48 mmol/mol) are considered as being diabetics. Measurement of HbA1C is an indirect measure of average blood glucose levels and need to include other factor into consideration which can affect hemoglobin glycation independently, like hemodialysis, pregnancy, HIV treatment, age, race/ethnicity, pregnancy status, genetic background and anemia/hemoglobinopathies. In general, FPG, 2-h PG during 75-g OGTT and A1C are equally appropriate for diagnostic screening of diabetes [10].
Classification of diabetes mellitus
DM is a broad term given for a group of diseases that are characterized by prolonged and persistent increase in blood sugar level. Even though, the classification of diabetes is based on the difference in the cause of development of the disease but many people with diabetes are not categorized under single type of diabetes. Recent advances in understanding the disease pathophysiological pathways and an emerging technology for disease detection has contributed for revisiting the classification of diabetes. Currently diabetes has been classified into two major classes: These Include Type-1 Diabetes (T1DM) and Type-2 Diabetes (T2DM). This classification of diabetes is based on the age of onset, the need of insulin for treatment, degree of loss of β cell function, degree of insulin resistance and presence of diabetes-associated autoantibodies [11,2]. But, none of these differentiate one type of diabetes from the other due to the occurrence of obesity at a younger age, the prevalence of T1DM in adult hood, the need of administration of exogenous insulin for T2DM patients and the occurrence of T2DM in young age. In addition the progress in diagnosis of diabetes by molecular genetics is a basis for the classification of diabetes. Accordingly diabetes has been classified in to different categories like T1DM, T2DM, gestational diabetes, monogenic diabetes and hybrid types of diabetes to mention some. Such clear classification provides practical guidance to clinicians for assigning a type of diabetes to individuals at the time of diagnosis [12].
T1DM, also known as insulin dependent diabetes or juvenile onset diabetes with a characteristic feature of absolute deficiency of insulin is caused by autoimmune destruction of pancreatic β cells by autoantibodies, activated CD4+ and CD8+ T cells as well as macrophages infiltrating in to the pancreatic islets. The onset of T1DM usually occurs in childhood and early adulthood <35 years with genetics and environment are considered as contributing factors for its occurrence [13].
The most common type, T2DM is previously referred to as non-insulin dependent diabetes, type 2 diabetes, or adult-onset diabetes, which is caused by various degrees of β-cells dysfunction and insulin resistance and includes individuals who have insulin resistance and usually have relative insulin deficiency. T2DM is commonly associated with overweight and obesity. It may require exogenous insulin injections when oral medications cannot properly control the blood glucose levels [5,14].
Stem cells
Stem cells are defined as unspecialized cells that are capable of self-renewal and differentiation into a specific cell lineage. Stem cells are undifferentiated cells that can self-renew indefinitely and are able to give more mature cells with specialized functions like muscle cells, blood cells, nerve cells and other cell types in the body. Stem cells are highly specialized cells that are capable of maintaining the injured tissues and cells lost every day. Stem cells have basic properties like able to give rise at least one type of mature, differentiated cell and prolonged selfrenewal [15,16]. Stem cells are generally classified in to four different groups based on their origin and potency. Based on their source they are classified in to as Embryonic Stem Cells (ESC), fetal stem cells, adult stem cells and induced Pluripotent Stem Cells (iPSC) [17-19].
Embryonic stem cells
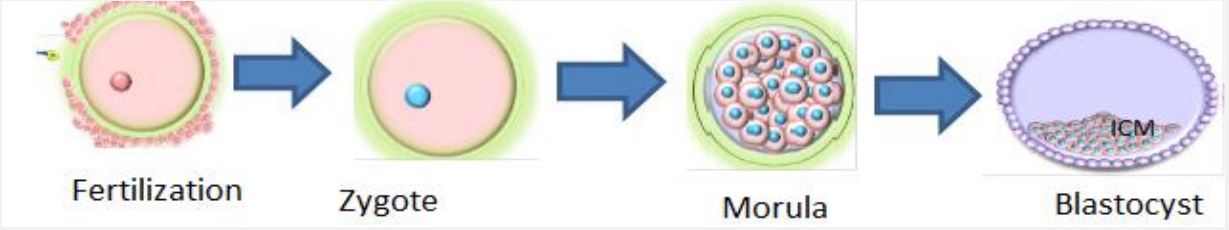
Embryonic Stem Cells (ESCs) are pluripotent cells derived from an early stage of embryo that form a hollow sphere of cells called a blastocyst. Blastocyst is an early cell mass of the developing embryo from 4th to 7th day off zygote formation that will disappear after the 7th day in normal embryonic development (Figure 2). The outer layer of the blastocyst is called as trophoblast with a cluster of cells inside the trophoblast is known as Inner Cell Mass. Embryonic stem cells are isolated from the inner cell mass of the early developing embryo (blastocyst-stage). Finally the trophoblast develops in to a placenta that provides nutrients to the embryo and the inner cell mass will develop to form all major tissue types; ectoderm, mesoderm and endoderm of the embryo. ESCs are extracted from the inner cell mass of the blastocyst stage, cultured in the laboratory under appropriate condition will proliferate indefinitely. ESCs growing in this undifferentiated state retain the potential to differentiate into cells of all three embryonic tissue layers [15,18].
Adult stem cells
Adult stem cells, which are also termed as somatic stem cells or resident stem cells (tissue specific stem cells), are a type of undifferentiated cells, resided in a differentiated tissue, organ or organism, in a specialized structural local microenvironment, called stem cell niche. The stem cell niche, is the in vivo microenvironment where stem cells are located and receive extrinsic signals interact and integrate to influence stem cell behavior that generally determine their fate [21,22]. The cellular niche is a specific cellular environment which can receive extrinsic signals and stimuli that can influence the cell behavior, like growth and development of stem cells. The stimuli induced by cell-tocell and cell matrix interactions may activate and/or repress genes and transcription programs. As a result of such interactions stem cells are maintained in a dormant state, induced to self-renewal or commit to a more differentiated state. Up on cell loss or tissue injury adult stem cells maintain tissue homeostasis through proliferation and differentiation to the required type of cells. Adult stem cells have been identified in many tissues including blood, intestine, skin, muscle, brain and heart [23]. According to different preclinical studies adult stem cells from different organs have the capabilities for the structural and functional regeneration of that specific organ. The safety, feasibility and the functional role of stem cell therapy has been observed in human patients in different clinical trials [22].
Mesenchymal stem cells
Mesenchymal Stem Cells (MSCs) are fibroblast like, spindle shaped multipotent cells with tri-lineage differentiation potential. These were first isolated from rat bone marrow by Friedenstein and his colleagues and later have been found in various other tissues. These cells are characterized on the basis of the presence of certain surface markers (CD105, CD29, CD73, CD90, HLA class I molecules) and absence of hematopoietic and endothelial markers (HLA class II molecules, CD34, CD45, CD31, CD14) [24]. These can be expanded easily and have a population doubling time of approximately 24-48h and can be expanded in culture for more than 60 doublings. Bone marrow is the most established source of MSCs which has been used for in vitro differentiation into cells of all three lineages. However, in last decade other less invasive sources of MSCs have also been explored including dental pulp, adipose tissue, umbilical cord etc. Earlier MSCs were supposed to secrete only cytokines and growth factors for the support of hematopoiesis. However, later these cells have been found to differentiate into various lineages including osteoblasts, chondrocytes, neurons, skeletal muscle cells, cardiac cells, hepatocytes etc. Additionally, they migrate to the site of injury and help in regeneration by secretion of various bioactive factors. By virtue of these features, MSCs have come up as a promising modality in human clinical stem cell therapies. Various clinical trials using bone marrow derived MSCs followed by adipose tissue and umbilical cord derived MSCs have been completed for diseases like Myocardial Infarct, Stroke, Diabetes, Spinal Cord Injury etc [25,26].
Induced Pluripotent Stem Cells (iPSCs)
IPSC are a type of pluripotent stem cell that can be generated directly from somatic cells through reprogramming. Using external stimulation factors terminally differentiated cells (somatic cells) can be reverted in to the state of pluripotency to generate iPSC. Most of iPS cells are derived by using four factors Oct3/4, Sox2, Klf4 and c-Myc and the iPS cells have been generated from most organisms including humans [27] Oct4 (octamer-binding transcription factor 4), Sox2 (SRY (sex determining region Y)-box 2), c-Myc (a bHLH/LZ (basic Helix-Loop-Helix Leucine Zipper) domain-containing oncogene, similar to myelocytomatosis viral oncogene (v-Myc)) and Klf4 (Kruppel-like factor 4) (OSCK or Yamanaka factors) by Shinya Yamanaka’s laboratory in Kyoto, Japan [28]. However, the process of obtaining iPSCs is expensive and time-consuming. Furthermore, the translational use of iPSCs has been limited due to the potential risk of oncogenesis and insertional mutagenesis, poor integration into host neuronal circuits and production of immune-tolerable cells [29,30].
Application of stem cells
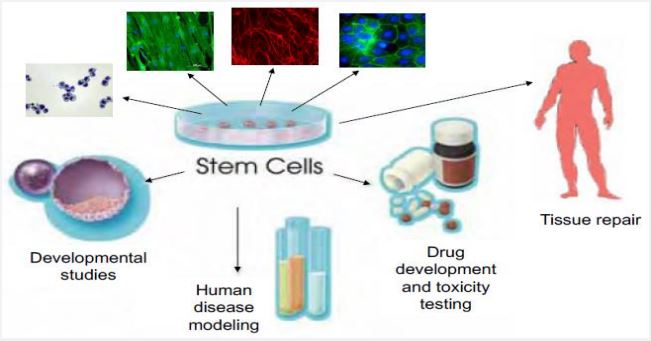
In regenerative medicine stem cells have a wide application in restoration of specific tissue parts (Figure 3). Some of their applications include; Improvement of spinal cord injury, Regeneration of retinal sheet, Generation of retinal ganglion cells, Healing of heart defects, Hepatic cell formation, Cartilage lesion treatment, in vitro gametogenesis, Regeneration of kidney tissue, Vision restoration in age related macular degeneration, Treatment of placental defects, Treatment of liver and lung disease, Treatment of diabetes and retinopathy, Neurodental therapeutic applications, Restoration of cognitive functions, Brain and cancer treatment, Ear acoustic function restoration, Regeneration of intestinal mucosa, Treatment of vision defects, Muscle regeneration, Regeneration of fallopian tube, Regeneration of bladder tissue, Treatment of anemia and blood cancer, Formation of insulin secreting 𝛽-cells [29].
Stem cells in the treatment of diabetes mellitus
T1DM which is caused by autoimmune mediated destruction of pancreatic islet cells accounts for 5-10% of diabetic patients. To prevent hyperglycemia associated complications, maintenance of appropriate glycemic control is mandatory and possibly glycemic control is done by using exogenous insulin for life and oral hypoglycemic drugs. Because of the inability to tightly control blood glucose levels within a normal physiological range, complications like life threatening episodes of hypoglycemia, micro- and macro-angiopathy that leads to cardiovascular pathologies, kidney failure and neuropathy. To prevent complications associated with long term administration of exogenous insulin, transplantation of purified human cadaveric pancreatic islets into the portal vein to replace the destroyed β cells of the patients or an intact pancreas is an ideal alternative for lifelong treatment [31]. However, due to the shortage of pancreatic donors and the need for drugs that suppress the immune system pancreatic transplantation becomes limited. As a result restoration or a tight regulation of insulin delivery through β-cell replacement represents the most promising approach in the treatment of type 1 diabetes [32]. The progress in the field of regenerative therapies provides the potential for the generation of surrogate ß-cells through engineering of InsulinProducing Cells (IPCs) from stem cells. Different studies were made to generate IPCs from stem cells; namely, ESCs, induced pluripotent stem cells (iPS cells) and MSCs, derived from a variety of adult tissues. This is due to the capability of stem cells to replace damaged cells in the body; therefore, they offer a promising treatment to replace the non-functional insulin-producing β cells of the pancreas [33].
Human Pluripotent Stem Cells (hPSCs), including human Embryonic Stem Cells (hESC) and induced Pluripotent Stem Cells (hiPSC), are considered very attractive alternative sources of surrogate β cells because of their ability to differentiate into all major somatic cell lineages. To date, the most success in producing pancreatic β-like cells from hPSCs has come from approaches that mimic normal pancreas development. Many research groups have followed this approach, which involves exposing the cells to various growth factors and signaling molecules [31]. For the differentiation of MSCs into insulin-producing cells in vitro, human MSCs were incubated with a series of solutions containing transcription factors and signal molecules such as β-fibroblast growth factor, epidermal growth factor, β cellulin and activin A. The study found that MSCs were differentiated into insulin-producing cells, which has been assessed by the presence of proinsulin and C-peptide in the solution [34,35].
Steps in the generation of IPCs from ESCs and iPSCs
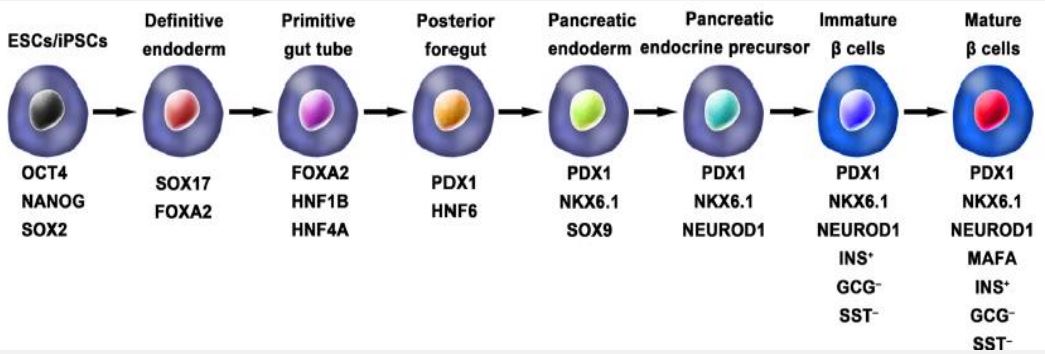
ESCs and iPSCs, have the capacity to proliferate and differentiate in to different cell types. As a result, hPSCs has a promising potential to generate in vitro insulin-secreting pancreatic beta cells. Using iPSCs derived from adult somatic cells through reprogramming, by using different stage specific transcription factors (Yamanaka factors) eliminates the ethical issues associated with the use of ESCs and graft rejections with tissue transplants. In general the stages in the generation of functional IPCs from hPSCs are based on the in vivo development of pancreas (Figure 4). The main steps in the development of embryonic pancreas involves the development of the Definitive Endoderm (DE), Primitive Gut Tube (PGT), Pancreatic Progenitor (PP), Endocrine Progenitor (EP) and hormone expressing endocrine cells. By adding a variety of cytokines for instance epidermal Growth Factor (bFGF) and signaling molecules like, bone morphogenetic proteins, γ-secretase inhibitors in each stage resulted in activation or inhibition of specific signaling pathways (e.g., Notch, Wnt) involved in the generation of adult β cells, the hPS cell fate is manipulated into pancreatic β cell phenotype [36,37].
In the year 2014 a breakthroughs comes from Rezania and colleagues report, in which a more detailed protocol for the generation of insulin producing cells from hPSCs that are comparable to human β cells. Accordingly the IPC generation protocol has 7 sequential stages, which include: stage-1; definitive endoderm, stage-2; primitive gut hub, stage-3; posterior foregut, stage-4; pancreatic endoderm, stage-5; pancreatic endocrine precursors, stage-6; immature β cells and stage-7; become maturing β cells (Figure 4). These cells show expression of mature β cell markers, such as MAFA, PDX1/NKX6.1 and INS with functional similarities to human islets after in vivo transplantation. These β-like cells rapidly reversed hyperglycemia in STZ induced diabetic mice evidenced by the presence of C-peptide and insulin. Even though the produced pancreatic beta like cells are not similar to human pancreatic beta cells and their response to glucose stimulation is not sharp [36-38].
Stem cell transplantation for the treatment of DM
Mesenchymal Stem Cells (MSCs) are defined as a fibroblastlike cell population capable of differentiating into multiple mesenchymal lineages in vitro, including bone, fat and Wharton’s jelly. MSCs possess the capacity to differentiate into islet-like Insulin Producing Cells (IPCs), to promote the regeneration of pancreatic islet beta cells and protect endogenous pancreatic islet beta cells from apoptosis through immunomodulatory mechanisms [39,40].
In different animal based studies MSC transplantation has been found as a successful method to treat hyperglycemia associated with type 1 diabetes. MSCs which are found in nearly all organs and tissues were multipotent stem cells that can be differentiated into a variety of cell types. Such properties allow MSCs as commonly used in tissue repair for a variety of conditions. A study performed by Aali and colleagues in 2014 tested therapeutic effects of MSC on diabetic rats. For the study they have used Male Wistar rats by dividing them into five groups as; normal control, diabetic control, MSC-treated, supernatanttreated and MSC- and supernatant-treated. The supernatant in this experiment came from the medium in which MSCs were grown. Over the course of several weeks, the MSC- treated diabetic rats had reduced blood glucose levels and higher insulin levels compared to the diabetic control group. Supernatanttreated rats also had reduced blood glucose levels, but to a lower extent. The greatest improvement in glucose and insulin levels was seen in the MSC and supernatant-treated rats. Immuno-histochemical analysis of the pancreatic tissues of the rats revealed that MSC-treated, supernatant-treated and MSC and supernatant-treated rats had partially regenerated pancreatic tissues that can be evidenced by the observation of new and larger islets of Langerhans [35,41].
In a study done by Si et al., infusion of MSCs to a diabetic rat model resulted in a significant regeneration of endogenous beta cells like that has been observed by Aali et al. Moreover, the infusion of MSC significantly improved insulin sensitivity as evidenced by elevations in phosphorylated insulin receptor substrate-1 (IRS-1), protein kinase B (Akt) and GLUT4 within insulin targeted tissues [42]. In a similar study conducted by Lee and colleagues infusion of BM-MSCs resulted in the regeneration of mouse pancreatic beta cells [43]. Moreover MSCs are able to protect pancreatic islet beta cells through immuno-regulation which is considered as the major mechanism in which MSCs exert their antidiabetic effect. MSCs are believed to prevent the autoimmune destruction of insulin producing pancreatic beta cells in type-1 DM through; suppression of T cell responses to mitogenic and antigenic stimulation, inhibition of dendritic cell differentiation and inhibition of B cell proliferation in a dosedependent manner [44].
MSCs’ ant oxidative and ant apoptotic effects has been enhanced by the amplified production of anti-inflammatory cytokines like TGF-β and TNF-α that played an important role in the protection of endogenous pancreatic islet cells. In the same analysis, MSCs also showed a capability to reduce total reactive oxygen species, nitric oxide and superoxide ions; to downregulate Caspase-3, Caspase-8, p53; and to upregulate Bcl2, thereby confirming MSCs’ ant apoptotic properties. Diabetic hyperglycemia often leads to oxidative stress injury which further exacerbates the progression of diabetes. Therefore the ant oxidative and ant apoptotic capacity of MSCs may further promote pancreatic islet cell survival and thus slow or at least prevent the deterioration from impaired glucose tolerance to T2DM.
Numerous MSC-released factors such as; Transforming Growth Factor-β1 (TGF-β1), Indole amine 2,3-Dioxygnase (IDO), Nitric Oxide (NO), Human Leukocyte Antigen-G (HLA-G), Prostaglandin E2 (PGE2), Interleukin-1 Receptor Antagonist (IL-1RA) and Tumor Necrosis Factor-Stimulated Gene 6 (TSG-6) exhibit potent immunomodulatory characteristics [43,45]. Due to these and other cytokines, MSC have been described to induce regulatory T cells and anti-inflammatory M2 macrophages. More over MSC inhibit T cells, natural killer cells and T helper (Th) 17 cell differentiation as well as dendritic cells maturation [46]. The immuno-regulatory properties of MSCs are therefore believed to be vital to the restorative effects observed in both T1DM and T2DM patients treated with MSCs.
According to Xin and colleagues study mice transplanted with IPCs, the blood glucose levels of the diabetic mice was normalized within 6 days and maintained in the normal range throughout the observation period of 21 days. But mice treated with no cells or undifferentiated hMSCs remained hyperglycemic. The cells are transplanted in to the renal sub-capsular space showed positive for insulin and c-peptide formation with no obvious IPCs apoptosis. From such kind of finding it has been strongly indicated that hMSC-derived IPCs could effectively control hyperglycemia in diabetes [47].
In animal models of T1DM, MSCs can ameliorate or reverse the manifestation of diabetes. Carlsson and colleagues study demonstrated that MSC treatment could preserve β cell functions in new-onset T1DM patients. For their study, they have used twenty adult patients (aged 18-40 years) with newly diagnosed (<3 weeks) T1DM and enrolled as MSC treatment group or to the control group and followed for 1-year. At the end of the clinical trial, Mixed-Meal Tolerance Tests (MMTTs) revealed that both C-peptide peak values and C-peptide significantly increased in the treatment group with no MSC side effects. In response to the MMTT, patients in the control arm had a mean decrease in both C-peptide peak values and C-peptide when calculated as area under the curve during the 1st year [48]. A study conducted by Cai and colleagues on 42 patients aged 18- 40 years with a history of T1DM for ≥2 years and ≤16 years. The study participants were randomized into two groups as stem cell transplant receivers (umbilical cord MSCs in combination with autologous bone marrow mononuclear cells) or standard insulin care treatment groups. After a 1-year follow-up examination the C-peptide increased from 6.6 to 13.6 pmol/mL/180 min in treated patients, but it decreased from 8.4 to 7.7 pmol/ mL/180 min in control groups; insulin increased from 1477.8 to 2205.5 mmol/mL/180 min in treated patients; and it decreased from 1517.7 to 1431.7 mmol/mL/180 min in control patients. Additionally, HbA1c and fasting glycemia decreased in the treated groups and increased in the control subjects. Daily insulin requirements in the treated groups also decreased compared to those of the control groups. During the follow-up period, severe hypoglycemic events reported by patients were significantly decreased. Limitations of these studies could be a small sample size and the short follow-up period [49,50].
In a follow up study done by Hu et al., on 29 DM patients; by grouping them in to two groups as MSC treatment group and control group. The treatment group has been given fresh human UC-MSCs via Intravenous infusion and SC-treatment was found to be safe. In the treated groups HbA1c was found to be lower and C-peptide was found to be higher in MSC-treated patients as compared to saline-treated patients [51].
In other study done by Oh and colleagues, differentiated bone-MSCs expressing pancreatic genes exhibited glucose responsive insulin secretion. The BMSCs aggregates has been transplanted in to sub-capsular renal region of hyperglycemic mice has lowered circulating blood glucose levels and maintained normal glucose levels for up to 90 days after transplantation [52]. Moreover islet like clusters formed from BMSCs has showed expression of multiple pancreatic genes with the ability to release insulin up on glucose stimulation. The effect of such cells has been shown in streptozotocin-induced diabetic mice by reducing hyperglycemia and improving metabolic profiles in response to glucose tolerance testing [53].
In the clinical as well as pre-clinical studies, administration of MSCs has been found to restore normoglycemia in diabetics even though their therapeutic mechanism was poorly understood. Their regenerative potential mainly involves, transdifferentiation, immunomodulation, apoptosis prevention and proliferation/differentiation induction. Even if in their in vitro differentiation MSCs have been reported to differentiate into beta islet cells and have the capacity to restore normoglycemia upon transplantation in diabetic animals, there in vivo differentiation of these cells into beta islet cells is still skeptical due to very low level of functional MSCs were found in transplanted pancreas [54].
In a randomized clinical trial study by using HSC and MSC for 12 months follow up period, the treatment result showed that an increase in C-peptide level in patients receiving HSC, MSC and MSC+HSC groups when compared with that achieved by conventional insulin treatment group. The higher C‑peptide levels significantly proved the regeneration of β‑cell function after SC therapy. Moreover the reduction of HbA1C levels has been observed in a MSC+HSC combined therapy which was significantly better efficacy when compared to insulin and MSC treatment. HSC, MSC and MSC+HSC all reduced the HbA1C levels over a period of 12 months after SC transplantation, whereas the UCB was not effective [55].
A reduction in inflammatory activity and insulin sensitivity was observed when UC-MSCs were infused into type 2 diabetic rats with a significant improvement in hyperglycemia. Similarly, infusion of Adipose-Derived MSCs (AD-MSCs), into diabetic NOD mice has been showed in the reversal of hyperglycemia through inducing higher serum insulin, amylin and glucagon-like peptide-1 levels compared to untreated controls. Mice treated with AD-MSC showed a reduction in CD4+ T helper (Th) 1 cells, interferon-γ and inflammatory cell infiltration [56]. According to Shigemoto-Kuroda and colleagues study administration of MSCderived EVs might induce IL-10-expressing regulatory DCs and thereby, regulatory DCs subsequently suppress Th1 and Th17 cell development without inducing Tregs which might inhibit the onset of type 1 diabetes [57]. This is due to the reduction in Th1 cytokine and IL-17A and/or IL-17F production which are responsible for the development of organ specific autoimmune diseases and inflammation in many disorders especially in autoimmune disorders. As a result MSC-derived EVs might be beneficial for treating autoimmune diseases where Th1 and Th17 cells play a critical role [57]. Different studies have reported the immunomodulatory properties of Bone Marrow-Derived Mscs (BM-MSCs) in islet xenotransplantation, as evidenced by reduced inflammatory markers and increased immune tolerance markers, demonstrating the potential of this strategy in solving transplantation issues of immune-related graft rejection [58].
To assess the safety and efficacy of autologous BM-MNCs and BM-MSCs for the treatment of T2DM a study was conducted on thirty patients who were on triple oral anti-diabetic drugs with insulin by dividing them into three groups. Accordingly, 20 patients were assigned in to two therapy groups, as group-1, 10 patients who were given BM-MNCs at a dose of 1x109 /kg, and group-2 also with 10 patients who were given BM-MSCs at a dose of 1x106 /kg and infused in to the direction of pancreas. The remaining 10 patients who are given placebo were assigned in the control group. In 12 months of follow-up period insulin requirement has been decreased more than by 50% in both therapy groups. C-peptide values have shown a significant increase in the treated group-1 patients and an increase in IRS-1 gene expression with significant improvement in insulin sensitivity in group-2 patients. From the study six patients who received MSCs showed a significant Weight reduction. No significant adverse side effects have been observed in both groups as a result it can be concluded that transplantation of MSCs and MNCs for T2DM is safe and effective. From the study, it’s recommended that giving both MNC and MSC may lead to a better outcome in glycemic control. The major limitation of the study was using a small sample size and a short follow-up period [59].
Stem cells from WJ-MSCs were differentiated into IPC and when transplanted into the liver has shown the expression of insulin, secretion of C-peptide and Expression of pancreasspecific genes [19]. In a clinical study long-term efficacy of IV administration of WJ-MSCs in 15 new onset type 1 diabetic patients was assessed by Hu and colleagues. They followed up the patients for their insulin requirements and HbA1C and Cpeptide levels for 24 months after transplantation. Three patients became insulin independent at the end of the follow up period. In other patients, insulin requirements and HbA1C levels decreased significantly in comparison to the control group. Moreover, C-peptide levels increased significantly in patients undergoing WJ-MSCs transplantation. They did not report any acute or chronic side effects or ketoacidosis in transplantation group while in control group ketoacidosis appeared in 3 patients [51,60].
According to the study by phadnis et al., on NOD/SCID mice which develop diabetes through pancreactomy and STZ administration; IPC derived from BM-MSC are transplanted into renal capsule and their effect has been studied for 70 days. The results of the study indicated that transplanted IPCs could mature and secrete human c-peptide in vivo and able to normalize blood glucose levels and stayed normal for up to 8 weeks thereafter. The IPC grafted mice showed weight gain, but the diabetic mice has continued weight loss with 50% mortality by day 40 after the onset of diabetes. After removal of grafted IPC, mouse blood glucose level increased to high levels within 3 days and 93% mice died within 3 weeks; no detectable human C-peptide in plasma; and all mice had BG levels above 350 mg/dL at 2 h of glucose challenge [61,62].
An interesting aspect in the treatment of diabetes is ‘Stem Cell Educator’ therapy. The ‘Stem Cell Educator’ therapy approach routed the patient’s blood through a closed-loop system that separates lymphocytes from the whole blood. The device is made by a stack of specially designed Petri dishes with adherent CB-SCs, functions as part of a closed-loop system that circulates a patient’s blood through a blood cell separator. In this procedure the lymphocytes were separated from the blood while returning the other blood components to the patient. The separated lymphocytes were briefly co-cultured with adherent cord blood-derived MSC before returning them into the patient’s circulation. Through secreted and cell-surface signaling molecules, the CB-SCs educate the lymphocytes passing through the device. In this process MSC are not delivered into the body but their temporary contact to patient’s lymphocytes was sufficient to induce immune tolerance which ameliorates the disturbed Th1/Th2/Th3 cytokine balance with increased Treg numbers in type-1 diabetic patients and decreased CD86+/ CD14+ monocytes and reduced markers of inflammation in type 2 diabetic patients [63]. As a result, all patients displayed a generally reduced requirement for insulin and metformin and improved HbA1C values after 10-12 months follow-up. In addition, Homeostasis Model Assessment of insulin resistance (HOMA-IR) demonstrated that insulin sensitivity was improved post-treatment in type 2 diabetics. The potential of the ‘Stem Cell Educator’ is further investigated by 2 clinical trials. Stem Cell Educator therapy in T1D patients is safe and effective. Stem cell educator therapy increases cytokine production like TGF-β1 which is able to induce peripheral immune tolerance that can prevent β cells from infiltrating lymphocytes through ring formation around pancreatic islets to generate safe environment for β cell regeneration [63,46].
Conclusion
Still this day’s diabetes mellitus has no cure and the treatment options used were lifelong. As a result searching new treatment options becomes necessary and needs attention. One of the possible therapeutic approaches to achieve this goal is development of stem cells that can produce insulin. Stem cell based therapy for the treatment of diabetes has been considered as promising alternative in the cure of the disease. For the successful generation of glucose responsive pancreatic like insulin producing cells different researchers were trying to produce stem cells using crucial factors that mimic similar signaling mechanisms seen in vivo. Such trials have been done by adding those factors to the cell culture medium that gives a promise for production of insulin producing cells. Hence, due to their ease of isolation, immunomodulatory and tissue regenerative properties and the supportive niche they provide by secreting micro-environmental factors and deposition of extracellular matrix, MSCs are suggested to be a suitable stem cell resource for deriving in vitro β cells and for immunomodulation that may prevent graft rejection and autoimmune destruction of β cells. Moreover, stem cell educator therapy will also provide substantial improvements in the treatment of type 1 diabetes without the need for immune suppression.
References
- Mekala K, Bertoni A. Epidemiology of diabetes mellitus. (Eds: Giuseppe Orlando, Lorenzo Piemonti, Camillo Ricordi, Robert J Stratta, Rainer WG Gruessner), Transplantation, Bioengineering and Regeneration of the Endocrine Pancreas; Academic Press. 2020; 49-58.
- Karamanou M, Protogerou A, Tsoucalas G, Androutsos G, Poulakou-Rebelakou E. Milestones in the history of diabetes mellitus: The main contributors. World J Diabetes. 2016; 7(1): 1-7.
- Lin X, Xu Y, Pan X, Xu J, Ding Y, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Scientific reports. 2020; 10(1): 14790.
- Cho H, Shaw E, Karuranga S, Huang Y, Da Rocha Fernandes D, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018; 138: 271-281.
- WHO. Classification of diabetes mellitus. Geneva; Licence: CC BY-NC-SA 3.0 IGO. 2019.
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes research and clinical practice. 2019; 157: 107843.
- Chen H, Chen G, Zheng X, Guo Y. Contribution of specific diseases and injuries to changes in health adjusted life expectancy in 187 countries from 1990 to 2013: Retrospective observational study. BMJ. 2019 Mar 27; 364.
- International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019; 157: 107843.
- Yau M, Maclaren NK, Sperling MA. Etiology and Pathogenesis of Diabetes Mellitus in Children and Adolescents. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc. 2021; 2000. https://www.ncbi.nlm.nih.gov/books/NBK498653/
- American Diabetes Association. 2 Classification and diagnosis of diabetes: Standards of Medical Care in Diabetesd2021. Diabetes Care. 2021; 44(1): S15-S33.
- Leslie R, Palmer J, Schloot N, Lernmark A. Diabetes at the crossroads: Relevance of disease classification to pathophysiology and treatment. Diabetologia. 2016; 59:13-20.
- Banday MZ, Sameer AS, Nissar S. Pathophysiology of diabetes: An overview. Avicenna journal of medicine. 2020; 10(04): 174-88.
- Kaul K, Tarr J, Ahmad S, Kohner E, Chibber R. Introduction to diabetes mellitus. Adv Exp Med Biol. 2012; 771: 1-11.
- Cheng SK, Park EY, Pehar A, Rooney AC, Gallicano GI. Current progress of human trials using stem cell therapy as a treatment for diabetes mellitus. American Journal of Stem Cells. 2016; 5(3): 74.
- Chagastelles P, Nardi N. Biology of stem cells: An overview. Kidney inter, Suppl. 2011; 1: 63-67.
- Gepstein L. Derivation and potential applications of human embryonic stem cells. Circ Res. 2002; 91(10): 866-76. doi: 10.1161/01.res.0000041435.95082.84.
- Sng J, Lufkin T. Advances in Stem Cell Therapies. Eds Bhartiya B, Lenka N, Intech. Janeza Trdine 9, 5100 Rijeka, Croatia. 2013; 375-96.
- Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration 2013; 85(1): 3-10.
- Babiker NE, Gassoum A, Abdel Raheem NE, Arbab MA, ALDeaf SA, et al. The progress of stem cells in the treatment of diabetes mellitus type 1. Progress in Stem Cell. 2017; 4(01): 175-88.
- Colaco S, Sakkas D. Paternal factors contributing to embryo quality. Journal of Assisted Reproduction and Genetics. 2018; 35: 1953-68. doi: 10.1007/s10815-018-1304-4.
- Ferraro F, Celso C, Scadden D. Adult stem cells and their niches. Advances in experimental medicine and biology. 2010; 695: 155-168. https://doi.org/10.1007/978-1-4419-7037-4_11
- Gurusamy N, Alsayari A, Rajasingh S, Rajasingh J. Adult Stem Cells for Regenerative Therapy. Prog Mol Biol Transl Sci. 2018; 160: 1-22.
- Bruyneel A, Sehgal A, Malandraki-Miller S, Carr C. Stem cell therapy for the heart: Blind alley or magic bullet? J Cardiovasc Transl Res. 2016; 9: 405-418.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315-7.
- Estrada EJ, Valacchi F, Nicora E, Brieva S, Esteve C, et al. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell transplantation. 2008; 17(12): 1295-304.
- Kang S, Shin M, Jung J, Kim Y, Kim C. Autologous adipose tissuederived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006; 15: 583-94.
- Okita K, Yamanaka S. Induced pluripotent stem cells: Opportunities and challenges. Philos Trans R Soc Lond B Biol Sci. 2011; 366(1575): 2198-207.
- Kawser Hossain M, Abdal Dayem A, Han J, Kumar Saha S, Yang G, et al. Recent Advances in Disease Modeling and Drug Discovery for Diabetes Mellitus Using Induced Pluripotent Stem Cells. Int J Mol Sci. 2016; 17(2): 256.
- Liu H, Reiter S, Zhou X, Chen H, Ou Y, et al. Insight In to the Mechanisms and the Challenges on Stem Cell-Based Therapies for Cerebral Ischemic Stroke. Front. Cell. Neurosci. 2021; 15: 637210.
- Singh V, Kumar N, Kalsan M, Saini A, Chandra R. Mechanism of Induction: Induced Pluripotent Stem Cells (iPSCs). J Stem Cells. 2015; 10(1): 43-62.
- Mahaddalkar P, Scheibner K, Pfluger S, Ansarullah, Sterr M, et al. Generation of pancreatic β cells from CD177+ anterior definitive endoderm. Nat Biotechnol. 2020; 38(9): 1061-1072.
- Efrat S. Generation of surrogate beta cells from tissue stem cells. Curr Diab Rep. 2004; 4(4): 298-303.
- Liu X, Wang Y, Li Y, Pei X. Research status and prospect of stem cells in the treatment of diabetes mellitus. Sci China. 2013; 56: 306-312.
- Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells’ generation from bone marrow-derived mesenchymal stem cells. J Diabetes Res. 2014; 2014: 628591. doi: 10.1155/2014/628591.
- Aali E, Mirzamohammadi S, Ghaznavi H, Madjd Z, Larijani B, et al. A comparative study of mesenchymal stem cell transplantation with its paracrine effect on control of hyperglycemia in type 1 diabetic rats. J Diabetes Metab Disord. 2014; 13(1): 76.
- Chen S, Du K, Zou C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Research Therapy. 2020; 11(1): 1-3.
- Carvalho AM, Nunes R, Sarmento B. From pluripotent stem cells to bioengineered islets: A challenging journey to diabetes treatment. European Journal of Pharmaceutical Sciences. 2022; 172: 106148.
- Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature biotechnology. 2014; 32(11): 1121-33.
- Chandravanshi B, Bhonde R. Shielding engineered islets with mesenchymal stem cells enhance survival under hypoxia. J Cell Biochem. 2017; 118: 2672-2683.
- Cho J, D’Antuono M, Glicksman M, Wang J, Jonklaas J. A review of clinical trials: Mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am J Stem Cells. 2018; 7(4): 82-93.
- Meirelles Lda S, Fontes A, Covas D, Caplan A. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009; 20: 419-427.
- Si Y, Zhao Y, Hao H, Liu J, Guo Y, et al. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: Identification of a novel role in improving insulin sensitivity. Diabetes. 2012; 61: 1616-1625.
- Lee R, Oh J, Choi H, Bazhanov N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. Journal of cellular biochemistry. 2011; 112: 3073-8.
- Pagliuca F, Millman J, Gurtler M, Segel M, Van Dervort A, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014; 159: 428-39.
- Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS, Kalionis B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta. 2017; 59: 87-95.
- Päth G, Perakakis N, Mantzoros C, Seufert J. Stem cells in the treatment of diabetes mellitus - Focus on mesenchymal stem cells. Metabolism. 2019; 90:1-15.
- Xin Y, Jiang X, Wang Y, Su X, Sun M, et al. Insulin-Producing Cells Differentiated from Human Bone Marrow Mesenchymal Stem Cells In Vitro Ameliorate Streptozotocin-Induced Diabetic Hyperglycemia. PLoS One. 2016; 12; 11(1): e0145838. doi: 10.1371/journal.pone.0145838.
- Carlsson P, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015; 64(2): 587-92.
- Cai J, Wu Z, Xu X, Liao L, Chen J, et al. Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care. 2016; 39(1): 149-57.
- Hwang G, Jeong H, Yang H, Kim H, Hong H, et al. Efficacies of Stem Cell Therapies for Functional Improvement of the β Cell in Patients with Diabetes: A Systematic Review of Controlled Clinical Trials. Int J Stem Cells 2019; 12(2):195-205.
- Hu J, Yu X, Wang Z, Wang F, Wang L, et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 2013; 60: 347-57.
- Oh S, Muzzonigro T, Bae S, LaPlante J, Hatch H, et al. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004; 84: 607-17.
- Kakkar A, Sorout A, Tiwari M, Shrivastava P, Meena P, et al. Current Status of Stem Cell Treatment for Type I Diabetes Mellitus. Tissue Eng Regen Med. 2018; 15(6): 699-709.
- Ezquer F, Ezquer M, Contador D, Ricca M, Simon V, et al. The antidiabetic effect of mesenchymal stem cells is unrelated to their trans differentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 2012; 30: 1664-74.
- Gan J, Wang Y, Zhou X. Stem cell transplantation for the treatment of patients with type 1 diabetes mellitus: A meta-analysis. Exp Ther Med. 2018; 16(6): 4479-4492.
- Lin H, Chan T, Fu R, Chuu C, et al. Applicability of adipose-derived stem cells in type 1 diabetes mellitus. Cell Transplant. 2015; 24(3): 521-32.
- Shigemoto-Kuroda T, Oh J, Kim D, Jeong H, Park S, et al. MSCderived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Reports. 2017; 8(5): 1214-25.
- Solis M, Velásquez I, Correa R, Huang L. Stem cells as a potential therapy for diabetes mellitus: A call-to-action in Latin America. Diabetology & metabolic syndrome. 2019; 11(1): 1-13.
- Hamad FR, Rahat N, Shankar K, Tsouklidis N. Efficacy of stem cell application in diabetes mellitus: Promising future therapy for diabetes and its complications. Cureus. 2021; 13(2). doi: 10.7759/cureus.13563.
- Hashemian S, Kouhnavard M, Nasli-Esfahani E. Mesenchymal stem cells: rising concerns over their application in treatment of type one diabetes mellitus. Journal of diabetes research. 2015. http://edelweissconnect.com/sites/default/files/bb/161028_BB_StemCells+Application_TSaric.pdf
- Phadnis S, Ghaskadbi S, Hardikar A, Bhonde R. Mesenchymal stem cells derived from bone marrow of diabetic patients portrait unique markers influenced by the diabetic microenvironment. The review of diabetic studies: RDS. 2009; 6(4): 260-270.
- Dang L, Phan N, Truong K. Mesenchymal stem cells for diabetes mellitus treatment: new advances. Biomedical Research and Therapy. 2017; 4(1): 1062-81.
- Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, et al. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012; 10:3.