Review Article
Volume 3, Issue 3
Reticulocyte Indexes and their Significance
Bashir Abdrhman*
Department of Hematology, Faculty of Medical Laboratory Sciences, Port Sudan Ahlia University, Sudan.
Corresponding Author :
Bashir Abdrhman
Email: bashirbashir17@hotmail.com
Received : Feb 20, 2024 Accepted : Mar 18, 2024 Published : Mar 25, 2024 Archived : www.meddiscoveries.org
Citation: Abdrhman B. Reticulocyte Indexes and their Significance. Med Discoveries. 2024; 3(3): 1135.
Copyright: © 2024 Abdrhman B. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
A crucial diagnostic tool for assessing, categorizing, and monitoring the effectiveness of anemia treatment is the reticulocyte count. The quality of erythropoiesis is an intrinsic aspect of the pathophysiology of anemia, and the ability of reticulocyte production would be one of the pivotal factors in the quality of erythropoiesis. However, in this minireview, we explain clearly whether the reticulocyte index’s ability is associated with clinical outcomes. There are several ways to describe the reticulocyte count: as a proportion of all red blood cells, as an absolute count, as a corrected count, as the Reticulocyte Production Index (RPI), or as the Immature Reticulocyte Fraction (IRF). Reticulocyte indexes aid in determining the etiology of anemia. They can distinguish between anemia-related causes and other dyserythropoietic problems.
Keywords: Reticulocyte; Reticulocyte count; RPI; ARC; IRF; CRC.
Introduction
Reticulocytes are premature erythrocytes created in the bone marrow and discharged into the peripheral circulation. Within one to two days, these cells develop into mature erythrocytes. Reticulocyte count shifts, particularly concerning anemias and bone marrow neglect, may indicate erythropoiesis activity or dysfunction [1].
As the reticulocyte evolves into a mature red blood cell, it goes through several structural modifications. An erythroblast’s chromatin and nuclear condensation take place in the skeletal marrow at the start of the process. Through interaction with macrophages, this mechanism facilitates enucleation, resulting in the formation of a reticulocyte. The endoplasm reticulum, Golgi apparatus, lysosomes, mitochondria, and ribosomes are among the organelles broken down and expelled by the reticulocyte during its time in the skeletal bone marrow. This process occurs through both autophagic and non-autophagic mechanisms. Ribonucleases aid in the degradation of RNA after it enters the circulation [2,3].
To create RBCs, some rRNA will still be present [2]. Exosomes are hypothesized to be engaged in changes in cell dimension and membrane remodeling. Since these modifications are selective, the proteins required to form mature, biconcave red blood cells are present throughout the reticulocyte’s life and can be released when needed [2,3].
Reticulocytes are larger, contain more hemoglobin, and have a lower hemoglobin concentration than a mature Red Blood Cell (RBC). Hemoglobin can only be produced by reticulocytes in the bone marrow. They therefore have the highest possible hemoglobin content after they reach the peripheral circulation [2].
A reticulocyte is an element of the erythropoiesis pathway. From an evolved hematopoietic stem cell, it develops [4]. Reticulocytes begin to construct in the bone marrow and mature there for one to three days. After that, they are liberated into the blood circulation, where they will eventually mature into mature normocytes in one to two days [5]. The reticulocyte goes through a lot of changes throughout this period to develop into a mature, functional RBC. Erythropoietin (EPO) levels rise, which causes the bone marrow to fabricate more reticulocytes. Before there is a noticeable rise in the reticulocyte number, EPO levels often rise for three to four days [1].
Juvenile normocytes (reticulocytes) are made up of strands of Ribosomal Ribonucleic Acid (RNA), which are more abundant in the cytoplasm of the nucleated precursors from which they originated. The characteristic of ribosomes is their ability to react with certain basic dyes and enable reticulocyte staging to differentiate, including azure B, brilliant cresyl blue, or new methylene blue, to produce a blue or purple precipitate of filaments or granules. This reaction happens exclusively in unfixed, vitally stained samples. Morphological traits can be used to identify different stages of maturity. The reticulocytes that have the most precipitable stuff are the most immature; the least immature have few spots or short strands visible. Four kinds of reticulocytes can be distinguished, from the most immature (group I) with a big clump of reticulin to the most mature (group IV) with a few reticulin granules. It is likely that when the cells leave the bone marrow, the circulation and especially the spleen experience a complete loss of basophilic material [6].
Assuming that reticulocytes are properly discharged from the bone marrow and circulate for the appropriate amount of time, the quantity of reticulocytes in peripheral blood is a pretty good indicator of erythropoietic activity. Because an increased erythropoietic stimulation causes an earlier release into the circulation, these presumptions are not always true. These socalled “stress” or stimulated reticulocytes can take up to three days on average to mature. In these circumstances, the proportion of immature reticulocytes in the blood will be higher than usual [2]. Quantitative flow cytometry of the reticulocytes’ RNA content allows for a more accurate evaluation of their maturity. However, a basic reticulocyte count that is reported as an absolute number per liter or, better yet, as a proportion of the red blood cells, usually provides sufficient information. When a person has severe anemia, their reticulocyte count should be reported as a reticulocyte index after being adjusted for the anemia [7].
Reticulocyte stains: New methylene blue produces better and more consistent outcomes than brilliant cresyl blue. Methylene blue, a weak reticulocyte stain, is chemically distinct from new methylene blue. Compared to brilliant cresyl blue, which varies in its staining capabilities from sample to sample, new methylene blue stains the reticulofilamentous material in reticulocytes more thoroughly and consistently. Azure B can be used as a suitable replacement for New Methylene Blue and has the benefit of being a pure dye that doesn’t deposit. It is applied with the same dose and staining technique as new methylene blue [8].
Staining solution preparation: In 100 milliliters of 3% trisodium citrate-saline solution (30 grams of sodium citrate in one liter of saline), dissolve one gram each of brilliant cresyl blue (CI 51010), new methylene blue (CI 52030), and azure B (CI 52010). When the dye has completely dissolved, filter.
Procedures
1. Mix equal amounts of blood and new methylene blue stain (2 to 3 drops, or approximately 50 µL each), and allow to incubate at room temperature for 3 to 10 minutes.
2. Remix the preparation.
3. Prepare two wedge films without fixing or counterstain.
4. In an area in which cells are close together but not touching, count 1000 erythrocytes under the oil immersion objective lens (1000X total magnification). Reticulocytes are implicated in the total RBC count
5. To boost accuracy, have another laboratorian count the other film; counts should concur within 20%.
6. Calculate the % reticulocyte count [8]:
Reticulocytes (%) = number of reticulocytes x 100 / 1000 (RBCs counted)
Important: Depending on the RBC, a precise amount of blood must be added to the dye solution for the best staining. It is recommended to add a higher volume of anemic blood and a lower volume of polycythaemic blood in comparison to normal blood. When the preparation is done correctly, the nonreticulated cells should be stained in various hues of pale greenish blue, whereas the reticulofilamentous stuff should be dyed deep blue. Counterstaining films are not recommended. After counterstaining, the reticulofilamentous matter is not more distinct, and precipitated stain-covering cells could be confusing. Furthermore, Heinz’s body will be invisible in fixed and counterstained arrangements. Under phase contrast, the stained preparation clearly defines the reticulocytes and mature red blood cells. With this method, cells with inclusion bodies can be easily separated from late reticulocytes, identified by filament or thread remains [6,8].
Counting reticulocytes: For the count, a section of the film with good staining and undisturbed cells should be selected. Making the film excessively thin is a typical mistake; however, the cells shouldn’t overlap. Use the 100 oil-immersion objective and, if available, eyepieces with an adaptable diaphragm (Miller ocular) to count the cells. Adequate spreading of the reticulocyte preparation is necessary to ensure an even distribution of cells in successive fields. The quality and resolving power of the microscope are also significant variables that affect the reliability of the count. The extremely accurate counts are performed by a vigilant expert who does not know the purported reticulocyte level, thus eliminating the effect of conscious or unconscious bias [8].
Absolute reticulocyte count: The exact number of reticulocytes in one liter (L) or one microliter (µL) of blood is known as the absolute reticulocyte count or ARC. A preliminary assessment of whether anemia is caused by insufficient or lost normocyte production can be made using the ARC [5,9].
ARC = reticulocytes (%) x RBCs count ( x 1012/L) / 1000
Corrected reticulocyte count: The proportion of reticulocytes in specimens that have minimal hematocrit may be falsely raised due to the lower concentration of normocytes in the entire blood. A correction factor is applied, with 45% for males and 42% for females being the average normal hematocrit.
corrected reticulocyte count (%) = reticulocyte (%) X patient HCT (%)/ 45
Important: To make up for the mild anemia, patients with a hematocrit of 35% should have an elevated corrected reticulocyte count of 2% to 3%. To make up for the mild anemia in individuals with a hematocrit of less than 25%, the count should rise to 3% to 5%. The extent of anemia affects the corrected reticulocyte count [6,8].
Reticulocyte production index: Shift reticulocytes are reticulocytes that are prematurely discharged from the marrow. These reticulocytes are “shifted” earlier than usual from the bone marrow to the peripheral circulation to make up for anemia. Unlike the majority of normal circulating reticulocytes, which shed their reticulum in a single day, these cells require two to three days to lose their reticula. If there is evidence of polychromasia in the red blood cell morphology, erythropoiesis evaluation should account for shift reticulocyte existence. Within a day of entering the bloodstream, the majority of normal (non-shift) reticulocytes mature into red blood cells, which means that they represent a day’s worth of red blood cell production in the bone marrow. Quickly shifted cells remain longer in the peripheral circulation as reticulocytes and provide a longer-lasting contribution to the reticulocyte count than one day. Consequently, in the presence of polychromasia, the reticulocyte count is falsely elevated since it no longer accurately reflects the cells growing in a single day. In numerous automated devices, the determination of immature reticulocyte fraction has taken the place of this mathematical modification of the reticulocyte count [1,2,6]. To calculate the proper correction factor (reticulocyte maturation time in days) (Table 1), the patient’s hematocrit is used:
Table 1: Correlation of Hematocrit with maturation time.
| Patient's Hematocrit value (%) | Correction factor (Maturation Time, Days) |
|---|---|
| 40-45 | 1 |
| 35-39 | 15 |
| 25-34 | 2 |
| 15-24 | 25 |
| <15 | 3 |
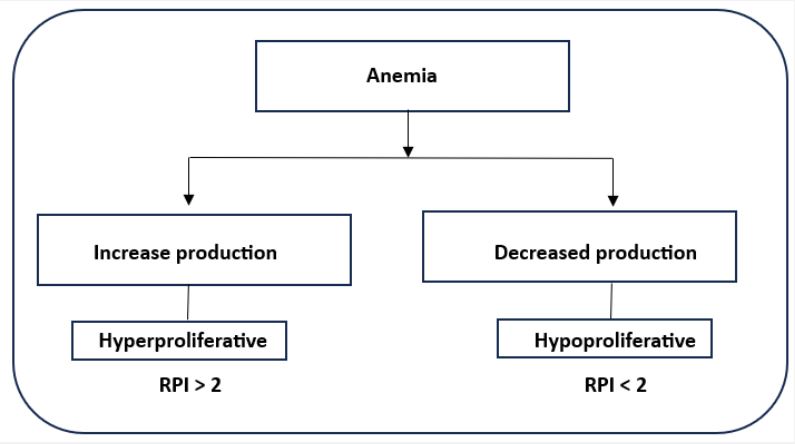
Generally speaking, an RPI of greater than 3 indicates a sufficient bone marrow response. When the RPI is less than 2, an insufficient erythropoietic response is observed, enabling the distinction between hyperproliferative and hypoproliferative anemia (Figure 1) [1,2,9]. The formula for calculating the reticulocyte production index (RPI) is as follows:
RPI= Corrected reticulocyte count / Maturation time
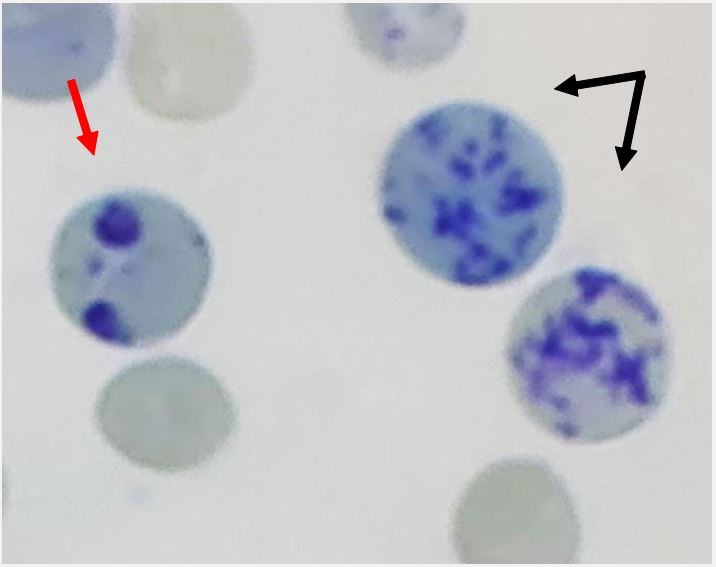
Distinguishing reticulocytes from other red cell inclusions: The fact that the most mature reticulocytes only contain a few spots or threads of reticulofilamentous material may make it challenging to determine what is and is not a reticulocyte. Luckily, Pappenheimer (iron-containing) granular material, which typically exists as a solitary, tiny dot or less frequently as multiple dots, stains a darker shade of blue than reticulofilamentous essence of the reticulocyte in well-stained preparations seen under a light microscope. To differentiate them, a phase contrast microscope will be useful. If in doubt, Perls’ response can be used to identify Pappenheimer bodies by overstaining the film for iron [9].
When bright cresyl blue or new methylene blue are present, hemoglobin H undergoes denaturation, producing rounded inclusion bodies that stain greenish-blue. These are easily distinguished from material that is reticulofilamentous. The reticulofilamentous material of reticulocytes is more strongly stained by methyl violet, whereas Heinz bodies stain with a milder shade of blue when stained with New Methylene Blue (Figure 2) [2].
Techniques for carrying out a reticulocyte count: Reticulocyte count measurements using light microscopy are no longer frequently performed. With a coefficient of variation varying from 25 to 48%, it is not very trustworthy and has low reproducibility [10]. Reticulocytes can be manually counted using fluorescence microscopy on films that have been suitably dyed. With electron microscopy, reticulocytes can be viewed in more detail, and changes in organization can be tracked throughout their lives. Although electronic counts are exact, accuracy must be ensured by careful consideration. Automated reticulocyte counts are provided by the leading instrument manufacturers. Every analyzer uses optical scatter or fluorescence to assess reticulocytes after treating red blood cells with fluorescent dyes or nucleic acid stains to stain any remaining RNA in the reticulocytes [9,10].
Reticulocyte indices: Some automated instruments provide additional reticulocyte parameters, such as the reticulocyte hemoglobin content and maturation index/Immature Reticulocyte Fraction (IRF) (which indicates the percentage of the more immature reticulocytes in the sample). When diagnosing early erythropoietic activity following chemotherapy or hematopoietic stem cell transplantation, the IRF may be very helpful. Reticulocyte hemoglobin can be used to identify iron insufficiency in its early stages [11].
Immature Reticulocyte Fraction (IRF): IRF is a numerical indicator of the reticulocytes’ RNA content. Compared to more mature reticulocytes, the quantity of RNA in immature (younger) reticulocytes is more extensive. Increased IRF, or reticulocytes with the highest RNA content, therefore implies early marrow reconstruction from conditioning regimens of stem cell transplantation, cancer chemotherapy, or treatment for nutritional anemias, and usually happens before a boost in absolute reticulocyte count [12]. In addition to assessing inefficient erythropoiesis, IRF has been used to distinguish between myelodysplasia (increased IRF) and megaloblastic anemia from other causes [3, 4].
The reticulocyte count and IRF can be combined to identify specific causes of anemia. For instance, reticulocytopenia with a high IRF suggests that the marrow is reestablishing, whereas a reduced absolute reticulocyte count accompanied by low IRF is linked to extreme aplastic anemia or kidney dysfunction. Lower than normal absolute reticulocyte count and increment IRF are linked to dyserythropoiesis, as demonstrated in a prompt reaction to iron prescription, whereas reticulocytosis with an excess IRF is usually observed in critical hemolysis or blood loss [13].
Reticulocyte hemoglobin content: Under the type of tool utilized, the automated hematology analyzer may additionally provide a measurement of reticulocyte-specific hemoglobin content as mean reticulocyte hemoglobin content (CHr) or Reticulocyte Hemoglobin equivalent (Ret-He). Two similar but distinct measures, CHr and Ret-He, provide an image of the functional iron that was accessible within Red Blood Cells (RBCs) for hemoglobin incorporation during the previous three to four days. A lower score reliably identifies functional iron insufficiency and typically indicates decreased cellular hemoglobin content. Moreover, this measure is the most reliable indicator of childhood iron deficiency anemia [11].
There are several circumstances when estimating the CHr or Ret-HE is helpful [4]. Serum ferritin can be mistakenly elevated as an acute-phase reactant despite low body iron storage, coupled with the physiologic variation of serum iron and total ironbinding capacity (the conventional iron panel is, therefore, less beneficial in these scenarios). This makes it challenging to diagnose functional iron deficiency in complex clinical contexts, such as chronic inflammation and chronic renal disease. May be a more accurate indicator of bone marrow iron reserves in nonmacrocytic individuals than conventional serum iron measures. When evaluating children and teenagers for iron deficiency before anemia develops, this marker is more accurate than hemoglobin [11].
Reticulocyte control: Currently, some commercial controls are available for tracking both automatic and manual reticulocyte counts. There are three levels for most of the controls. The treatment of the control samples is the same as that of the patient samples. When manual counts are carried out, the control can be utilized to confirm the precision and accuracy of the laboratorian [14].
Indication/applications: Reticulate counts are ordered and utilized in conjunction with Complete Blood Counts (CBCs) to guide anemia workup or treatment response; they are not typically included in normal CBC counts. The laboratory may report reticulocyte-specific hemoglobin content and IRF in addition to reticulocyte count, subject to the type of automated hematology analyzer being used. This can yield extra meaningful data [11,15].
Clinical interpretation of reticulocytes: Reticulocytes can be utilized as a beneficial clinical marker for anemias and the response of the bone marrow to anemia. The reticulocyte count will be low in anemic patients when the bone marrow is not functioning properly. Reticulocyte counts rise in response to suitable responses from the bone marrow [1]. Patients may develop reticulocytopenia as a result of [1,3]:
Hypochromic anemias: Because they generate a reduction in hemoglobin production, sideroblastic anemia, iron deficiency anemia, and anemia associated with chronic illness are all contributors to a decreased reticulocyte count.
Aplastic anemias: A pancytopenia originating from a failed bone marrow. Though there are numerous reasons for this, they all result in an impairment in erythropoiesis when reticulocytes develop in the bone marrow [16,17].
Dietary deficiency: Megaloblastic anemia is brought on by insufficient levels of folate and vitamin B12. The synthesis of DNA in the bone marrow depends on these nutrients, and their absence will result in a reduction in the formation of reticulocytes
Hemolytic anemia with an aplastic crisis: Aplastic crisis is a brief decrease in erythropoiesis brought on by a reduction in bone marrow precursors. In typical circumstances, an infection like salmonella, streptococcus pneumoniae, or parvovirus B19 causes this. When reticulocyte counts are inadequate in patients with hemolytic anemias, it may indicate an aplastic crisis.
Myelodysplastic syndromes: These forms of syndrome are characterized by a variety of bone marrow injuries. Typically, this affects the erythropoietic cell conformity, and the bone marrow cannot manufacture reticulocytes [16,17]. Reticulocytosis has the following causes [1].
Hemolytic anemias: These anemias are triggered via the breakdown of red blood cells. The ingredients needed to produce reticulocytes are still present in the bone marrow, which is still functional. It accelerates reticulocyte formation in response to anemia.
Blood loss: An increase in erythropoiesis will result from either acute or chronic blood loss.
Reaction to treatment: (Iron, vitamin B-12 or folic acid, erythropoietin, bone marrow recovery after chemotherapy, or bone marrow transplant/repair) [16,17].
Reticulocyte analysis, primarily Immature Reticulocyte Fraction (IRF), has also been employed to assess the bone marrow’s regeneration capacity before and after chemotherapy for leukemia patients. The same concept holdsfor recipients of stem-cell transplants [9,16].
Reticulocyte count is also used clinically in sickle cell anemia patients receiving hydroxyurea. This prescription medicine reduces the number by inhibiting the bone marrow’s ability to produce reticulocytes. Regular reticulocyte counts are therefore required while taking this medicine [18]. Reticulocyte count is currently applied as a measure of therapeutic response in patients with end-stage renal disease undergoing EPO therapy [1].
Considerations: Smokers typically develop secondary polycythemia, which is an elevated reticulocyte count accompanied by an increased normocyte count [7]. During the phase of adaptation to higher altitudes, the reticulocyte count or percentage briefly increases (temporary drop in the reticulocyte count or % following the adaptation to lower altitudes). As a result of the body adjusting to the increment in blood volume during pregnancy, the reticulocyte count might rise momentarily [7].
Takahashi et al. discovered in a different study that preterm children who are Slight for Gestational Age (SGA) had a minimized reticulocyte count than do preterm newborns who are not SGA, despite their earlier research suggesting that early preterm infants have greater reticulocyte counts [19].
Conclusion
Reticulocyte markers can assist in identifying the cause of anemia. They can discriminate between different dyserythropoietic issues and causes of anemia permitting the start of presumed treatment and maybe skipping intrusive procedures like bone marrow evaluation.
References
- Prchal JT. Production of Erythrocytes. Lichtman MA, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, eds. Williams Hematology. 8th ed. McGraw-Hill: New York. 2010; 31.
- Piva E, Brugnara C, Spolaore F, Plebani M. Clinical utility of reticulocyte parameters. Clin Lab Med. 2015; 35(1): 133-63.
- Moras M, Lefevre SD, Ostuni MA. From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Front Physiol. 2017; 8: 1076.
- Noulin F, Borlon C, van den Eede P, Boel L, Verfaillie CM, D’Alessandro U, Erhart A. Cryopreserved reticulocytes derived from hematopoietic stem cells can be invaded by cryopreserved Plasmodium vivax isolates. PLoS One. 2012; 7(7): 40798.
- Ryan DH. Examination of Blood Cells. Lichtman MA, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, eds. Williams Hematology. 2010. http://www.accessmedicine.com/content.aspx?aID=6106433. Accessed. 8th ed. New York: McGraw-Hill; January 15, 2012. Chapter 2.
- Marks PW, Glader B. Approach to Anemia in the Adult and Child. Hoffman F, Benz EJ, Shattil SJ, eds. Hematology: Basic Principles and Practice. 5th ed. Churchill Livingstone: Philadelphia, PA. 2009; 34.
- Briggs C, Bain BJ. Basic haematological techniques. Bain BJ, Bates I, Laffan MA, and Lewis SM eds. Dacie and Lewis Practical Haematology. 12th ed. Philadelphia PA: ELSEVIER. 2017; 3.
- D’Onofrio G, Zini G, Rowan RM. Reticulocyte counting: methods and clinical applications. In: Rowan RM, van Assendelft OW, Preston FE, eds. Advanced Laboratory Methods in Haematology. London: Arnold. 2002: 78-126.
- Clinical and Laboratory Standards Institute (CLSI). Methods for reticulocyte counting (automated blood cell counters, flow cytometry, and supravital dyes): approved guideline. (2nd ed.), CLSI document, Wayne, CLSI. 2004; 44-2.
- Means RT, Glader B. General Considerations. Greer JP, Foerster J, Rodgers GM, eds. Anemia: Wintrobe’s Clinical Hematology. 12th ed. Philadelphia, PA: Lippincott Williams and Wilkins. 2009; 1: 26.
- Hoffman R, Xu M, Finazzi G, Barbui T. The Polycythemias. Hoffman F, Benz EJ, Shattil SJ, eds. Hematology: Basic Principles and Practice. 5th ed. Philadelpha, PA: Churchill Livingstone; 2009; 68.
- Karagülle M, Gündüz E, Sahin Mutlu F, Olga Akay M. Clinical significance of reticulocyte hemoglobin content in the diagnosis of iron deficiency anemia. Turk J Haematol. 2013; 30(2): 153-6.
- Morkis IV, Farias MG, Rigoni LD, Scotti L, Gregianin LJ, Daudt LE, et al. Assessment of immature platelet fraction and immature reticulocyte fraction as predictors of engraftment after hematopoietic stem cell transplantation. Int J Lab Hematol. 2014. [QxMD MEDLINE Link].
- Briggs C, Bain BJ. Basic haematological techniques. Bain BJ, Bates I, Laffan MA, and Lewis SM eds. Dacie and Lewis Practical Haematology. 11th ed. Philadelphia PA: Churchill Livingstone. 2012; 3.
- Karen S. Clark and Teresa G. Hippel. Manual, Semiautomated, and Point-of-Care Testing in Hematology. In: Keohane EM, Walenga JM, Smith LJ. RODAK’S HEMATOLOGY: CLINICAL PRINCIPLES AND APPLICATIONS. 5TH ed. CANADA. PA: ELSEVIER. 2016; 14.
- Raja-Sabudin RZ, Othman A, Ahmed-Mohamed KA, Ithnin A, Alauddin H, Alias H, Abdul-Latif Z, Das S, Abdul-Wahid FS, Hussin NH. Immature reticulocyte fraction is an early predictor of bone marrow recovery post-chemotherapy in patients with acute leukemia. Saudi Med J. 2014; 35(4): 346-9.
- Das J, Khonglah Y, Tiewsoh I, Chowdhury Z, & Barman H. Utility of reticulocyte indices in the diagnosis of pancytopenia. Journal of Family Medicine and Primary Care. 2022; 11(4): 1335-1340.
- Vajpayee N, Graham SS, Bem S. Basic Examination of Blood and Bone Marrow. McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Elsevier/Saunders: Philadelphia, PA. 2011; 30.
- Agrawal RK, Patel RK, Shah V, Nainiwal L, Trivedi B. Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfus. 2014; 30(2): 91-6.
- Takahashi Y, Kanai Y, Chishiki M, Goto A, Imamura T. Neonatal reticulocytes among preterm infants of small for gestational age. Pediatr Neonatol. 2022. [QxMD MEDLINE Link].