Review Article
Volume 3, Issue 3
The View Point of Covid-19: Update Information
E Ghazizadeh1,2,3*; Zahra Naseri1
1Department of Bioinspired Materials and Biosensor Technologies, Institute of Materials Science, Faculty of Engineering, Kiel University, Germany.
2Radiation Sciences Department, Iran University of Medical Sciences (IUMS), Tehran, Iran.
3Department of Medical Biotechnology, Mashhad University of Medical Sciences, Mashhad, Iran.
Corresponding Author :
E Ghazizadeh
Email: elhamgenetic@yahoo.com
Received : Feb 12, 2024 Accepted : Mar 14, 2024 Published : Mar 21, 2024 Archived : www.meddiscoveries.org
Citation: Ghazizadeh E, Naseri Z. The View Point of Covid-19: Update Information. Med Discoveries. 2024; 3(3): 1132.
Copyright: © 2024 Ghazizadeh E. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
At the end of December 2019, “pneumonia of unknown cause” occurred Later, the specialized cause was identified and coined as Severe Respiratory Disorders Coronavirus 2 (SARS-CoV-2), and the disease was named “Coronavirus Disease 2019” (COVID-19). This recent new pollution brought the world to a standstill in a short period of time. On March 11, 2020, the WHO announced that the novel coronavirus disease is widespread. Analysts from around the world are coming together to study the pathogenicity and transmissibility of SARS-CoV-2 and derive treatments to curb this contamination. Analysts and researchers are implementing a unique expression of medicine in a comprehensive journey to find safer ways to control the obsessive effects of this viral infection. Numerous clinical studies are currently underway in the allopathic and naturopathy sector. Furthermore, global approaches to cell therapy and nanomedicine practices need to be optimized to obtain better clinical outcomes and useful results. Additionally, paradigm shifts in cell therapy and nanomedicine protocols will need to be optimized to achieve better clinical and functional outcomes for individuals affected by COVID-19. This article provides a comprehensive overview of the pathogenesis of the outbreak and various treatments to combat COVID-19.
Keywords: COVID-19; SARS-Cov-2; Pathogenesis; Diagnosis; Therapy; Vaccines; Therapeutics.
Introduction
At the end of winter in December 2019, the novel coronavirus emerged and its uncontrolled spread put the world at risk of becoming a widespread virus [1]. The etiological name of this infection was initially named 2019 novel coronavirus (2019- nCoV), but it was later renamed as severe respiratory disease coronavirus 2 SARS-CoV-2 and coronavirus disease 2019 (COVID-19). The increasing number of confirmed cases of COVID-19 presents a bleak picture of the current situation and requires advanced prevention and control measures [2]. Recent studies have shown that SARS-CoV-2, which is widely present in wastewater systems, can have an important impact on the transmission of COVID-19 in the environment [65]. The potential health concerns from direct waterborne exposure to this virus in wastewater are well documented. However, the aerosolization route should also be considered in this context, as it may be a more direct respiratory route when humans are exposed to SARS-CoV-2.
Aerosol viruses are often produced locally in buildings and on a larger scale during wastewater treatment or irrigation. Inhalation of virus-laden aerosols, generated by faulty plumbing and wastewater systems, is considered a potential route of transmission in a residential area in Hong Kong where 187 people are infected. The clinical spectrum and manifestations include the respiratory system bearing the brunt of the virus. COVID-19 disease; Regardless, damage to the cardiovascular system has been described in detail by several patients. Currently, there are no identified antiviral treatments for the treatment of SARSCoV-2 infection [3]. The core of treatment is to provide ongoing care appropriate to the complaints expressed with calmness. Indeed, it has been shown that treatment with a combination of recombinant Interferon (IFN) and ribavirin appears to limit the response to SARS-CoV-2 disease. With the flattening of the COVID-19 infection curve, immunotherapies including plasma and antibodies from convalescent patients have been proposed to address the current situations [4,5]. In addition, antibody techniques vary, including the use of inactivated infections, live attenuated infections, viral vector-based immunity, subunit antibodies, recombinant proteins, and immunostaining. DNA, has been evaluated in organisms and is being developed as a possible preventative technique.
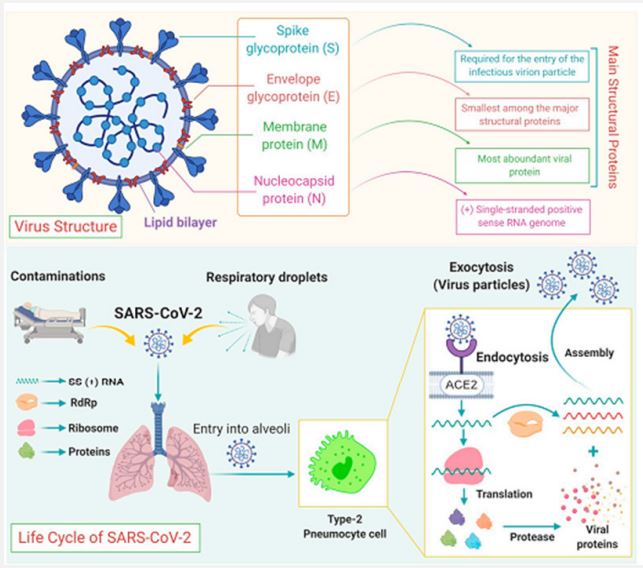
Due to the need for commonly licensed therapeutics or antibodies, contamination requires the use of contamination control measures with rapid, detailed implementation of successful drug containment and stabilization. Efforts to personalize by imparting solid knowledge of hand hygiene, respiratory hygiene, and hacking. behavior, wearing properly fitted protective gear and avoiding dense vegetation will have similar results. In this examination, we considered different haunting perspectives on the fusion, development, infectivity, and manifestation of COVID-19 [7,8]. In addition, we also highlight drugs and antibodies recently used in the fight against SARS-CoV-2 disease. Learning about COVID-19 is ongoing (Figure 1). This audit aims to summarize the initial results of the study on disease transmission, clinical highlights, conclusions, uses, and prevention of COVID-19 [9].
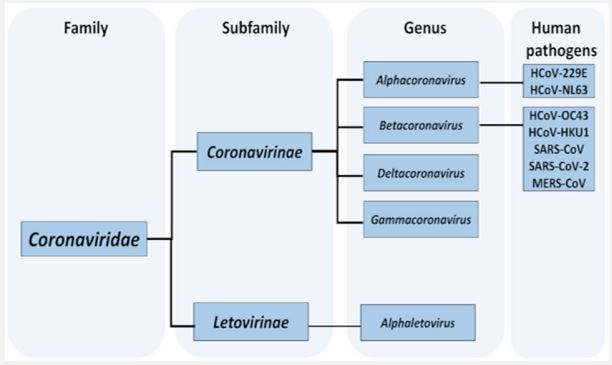
Taxonomy of COVID-19: Widespread infection of coronavirus disease 2019 (Covid-19) caused by coronavirus 2 (SARS-CoV-2), an extremely serious respiratory disorder, has prevailed in most most parts of the world, resulting in 129,471,273 confirmed cases and 2,825,407 deaths of April 1st 2021. COVID-19 was declared widespread worldwide on February 20, 2020 (WHO n.d.) [10]. Over the past two decades, five Coronaviruses (CoV) have been found to infect humans, namely SARS-CoV, human coronavirus NL63 (HCoV-NL63, 2004), human coronavirus NL63 (HCoV-NL63, 2004), human HKU1 (HCoV-HKU1, 2005), human coronavirus Oriental respiratory virus. Corona virus (MERS-CoV, 2012) and SARS-CoV-2 (2019), including SARS-CoV, MERS-CoV and SARS-CoV-2 are highly pathogenic CoVs. Along with the two human CoVs (HCoV-229E and HCoV-OC43) discovered in the 1960s, to date, seven CoVs have been found to be capable of infecting humans [11,12]. It is currently impossible to be certain about the animal origin of SARS-CoV-2, but it is clear that living creatures, including civets, foxes, minks and raccoons, all of which are susceptible to sarbecovirus infection, are being sold in Wuhan markets, including the Huanan display area (considered the epicenter of the outbreak in Wuhan) throughout 2019 [66]. Many of these creatures are raised on a large scale for their skins and are then sold in animal markets. Some of these farmed species (American minks, red foxes, and raccoon dogs) have been sold live for food by critters, as have wildcaught animals (including raccoon dogs and badgers). Despite the fact that no bats are used for food agreement. Together, this suggests a central role for living organisms susceptible to SARSr-CoV as the essential source of SARS-CoV-2 ancestors to which humans were exposed, as was the case with SARS [13].
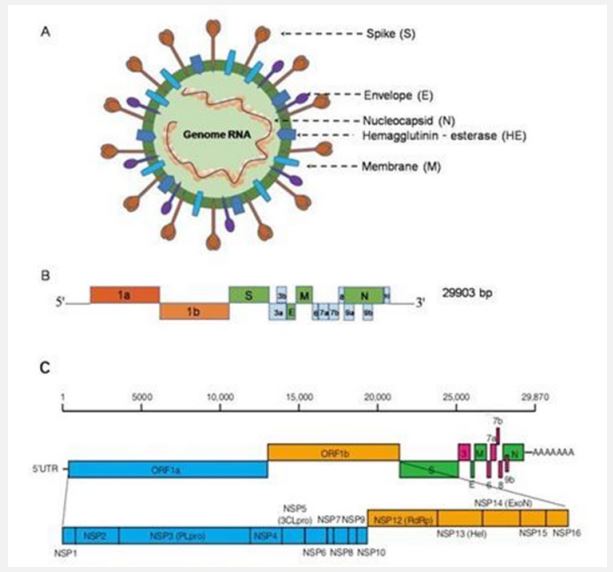
Structure of the Coronavirus genome: The SARS-CoV-2 genome consists of positive-sense single-stranded RNA. The recently sequenced SARS-CoV-2 genome was submitted to the NCBI genome database (NC_045512.2) with a size of ~29.9 KB [13]. The SARS-CoV-2 genetic cosmetic consists of 13 to 15 (12 extensions) Open Reading contigs (ORFs) containing approximately 30,000 nucleotides [14]. The genome contains 38% GC content and 11 protein coding features, of which 12 are reported proteins. The genetic process of ORF is deeply inspired by SARS-CoV and MERS-CoV [15,16]. The ORFs are organized into transcripts and proteases (1a-1b) and major proteins S, E, M, and N, which follow a commonly occurring 5′-3’ configuration and are considered major drug targets vaccine. These quality factors play an important role in the movement, fusion and survival of viruses in cells [17]. The genomic organization of SARS-CoV-2 has about 89% clustering characteristics with other CoVs. Decoded SARS-CoV-2 protein clusters were obtained from GenBank (promotion ID: NC_045512.2)]. The total genome of SARS-CoV-2 encodes nearly 7,096 long polyproteins including many accessory and Non-Structural Proteins (NSPs) (Figure 2) [18]. The nucleotide substance of the viral genome is essentially held by two nonstructural proteins ORF1a and ORF1ab, followed by accessory proteins. The polyproteins pp1a and pp1ab are encoded by ORFs 1a and 1b, of which polyprotein pp1ab is encoded by ribosomal frameshifter level 1b. These polyproteins are managed by virus-encoded proteinases and provide 16 proteins, which are well regulated in all CoVs of the same family. MERS-CoV is closely related to SARS-CoV-2 as it carries a larger genome of approximately 30,11 [19,20]. The genetic MERS cosmetic has a 5’ cap structure, a poly(A) tail at the 3’ end, the representative contains 16 NSPs numbered nsp1- nsp16 from the 5’ end. About 10 kb of the genome in the 3’ end constitutes the 4 basic traits (S, E, M, N) and 5 beauty proteins (ORF3, ORF4a, ORF4b, ORF5, ORF8) [21]. SARS-CoV-2 is generally more attractive than SARS-CoV and MERS-CoV, perhaps because of its unique epidemiology. It is perhaps conceivable that other mammals acted as “intermediaries” or “amplifiers” and that the resulting biological split could have caused some or all of the required changes essential for beneficial transmission to humans [22]. Comparative examination of SARS-CoV-2 gene clusters shows striking similarities to BAT-CoV [31]. Regardless, there is evidence that bats are a common reservoir of SARS-like CoVs, including SARS-CoV-2 [23,24]. CoV, which was requested halfway, was recently transmitted to humans. Additionally, a possible pangolin root of SARS-CoV-2 has also been suggested, based on the proximity of certain qualities [25]. It is still unclear how the bat CoV was genetically modified and transmitted to humans? Other evidence suggests that dogs are infected with SARS-CoV-2. It is curious to note that the human and canine Angiotensin-Converting chemical (ACE2) has a highly dispersive property (13 out of 18) and therefore the cause of the RBD peak of SARS-CoV-2 is the studies highly comparative, transmission recommended. between animals and humans [26].
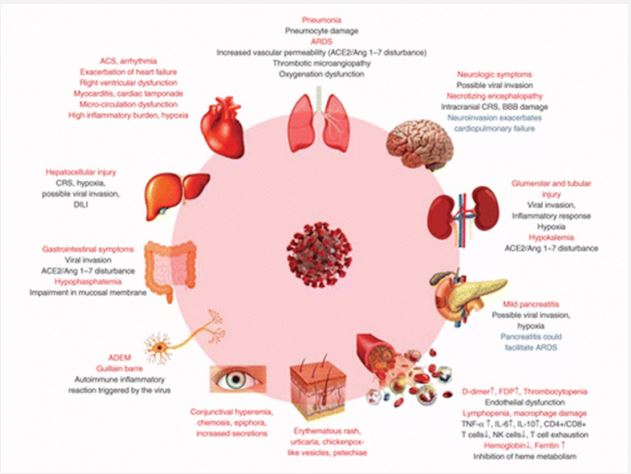
The clinical manifestations of COVID-19: Clinical manifestations of COVID-19 and clinical signs of COVID-19 vary in the general population. This reflection aims to systematize the article regarding the clinical aspects of patients confirmed to have COVID-19. An orderly study of the text was conducted. A total of 8,070 scientific products were found in the database [69]. Among the examinations, 184 patients met basic examination criteria, for a total of 114,046 patients. After comprehensive reading, 32 studies that did not report clinical signs were excluded. The final 152 distributions included a total of 41,409 people from at least 23 countries and 26 different clinical signs were detailed. Regarding the rate, 6 side effects have a greater prevalence or account for up to 25%, specifically fever (58.66%), dry cough (54.52%), and difficulty breathing (30.82). %), anxiety (29.75%), asthenia (28.16%), and phlegm/secretion (25.33%). Neurological indications (20.82%), dermatological signs (20.45%), anorexia (20.26%), myalgia (16.9%), wheezing (14.71%), sore throat. So, so to speak, think about the detailed dermatological aspects. The least frequently examined sign/ symptom was hemoptysis (1.65%). In a study of 100 patients, the top three side effects were fever (57.93%), dry cough (54.21%), and difficulty breathing (30.64%). Dermatological aspects were not among the most common side effects (Figure 3). Recognizable evidence of all clinical signs of COVID-19 is the basis for early identification and selection of preventive measures [70]. On the other hand, mucormycosis, commonly known as the dark organism, may be a rare but actually contagious disease caused by a fungus called mucormycete, which is abundant in the environment. It mainly affects people who have health problems or are taking medications that reduce the body’s ability to fight germs and disease. It was identified moderately as frequently as possible in Covid-19 patients in several Indian states. The infection usually occurs on the skin and also affects the lungs and brain. People who have been infected with COVID-19 and are still recovering have had their safety systems compromised, suggesting they are at greater risk because their bodies cannot fight off the contamination. People hospitalized for severe illness related to COVID-19 are likely to receive steroids to relieve their illness. Steroids work by reducing irritation in the lungs and reducing the body’s immune response to prevent it from attacking healthy cells in the body, leading to decreased levels of perceived safety. Steroids work by reducing inflammation in the lungs and dampening the body’s immune response to prevent it from attacking healthy cells in the body, leading to reduced immune surveillance. These patients are susceptible to mucormycetes. Although there has not been a large outbreak, the national COVID-19 task force has issued recommendations about this disease [71].
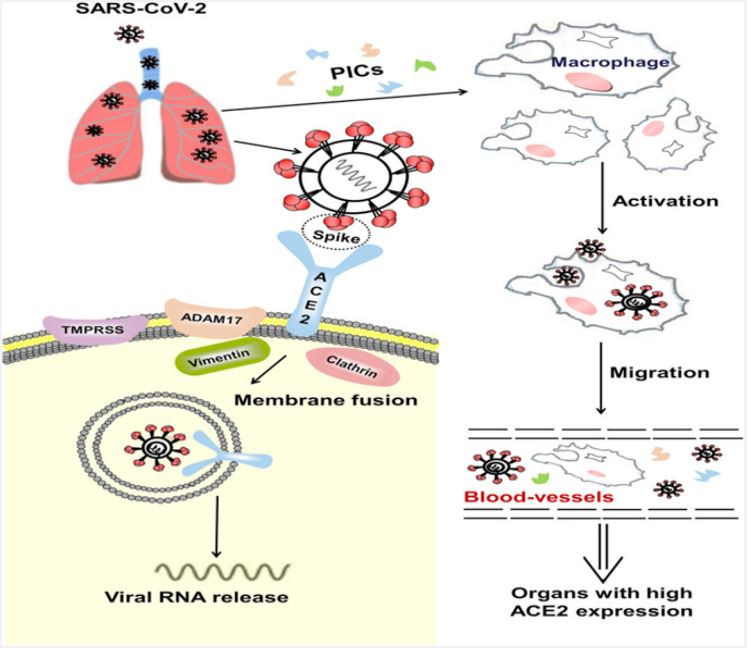
ACE2, a functional receptor of SARS-CoV-2: The angiotensinconverting chemical-2 may be a membrane-bound aminopeptidase, which is highly expressed in alveolar cells and plays an essential role in the cardiovascular system and resistance. It is included in improving heart function, hypertension and diabetes. By extension, ACE2 has been distinguished as a useful receptor for coronaviruses (including SARS-CoV and SARS-CoV-2; [27]). SARS-CoV-2 infection is caused by the viral envelope protein competent on the ACE2 receptor on the cell surface and mixing with the cell membrane, entering the cell through receptor-mediated endocytosis in a similar manner. similar to human immunodeficiency infections (HIV; [28]). It induces endocytosis of the ligand/receptor complex, triggering fusion between the infection and the infected cell and resulting in ACE2 being degraded inside the cell.
Since the epidemic SARS-CoV broke out in 2002, a large-scale baseline investigation at the nuclear level revealed that ACE2 could direct the inter-species and inter-individual transmission of SARS-CoV [29]. Improvements in surface plasmon resonance confirm that the exact strain between the membrane protein and ACE2 of SARS-CoV-2 is 10-20 times higher than that of SARS-CoV, suggesting that SARS-CoV-2 is more attractive than SARS-Co-V in genetic level [30]. This may also be the reason why SARS-CoV-2 spreads worldwide and depending on epidemiological developments, people of all ages can be infected. ACE2 is highly transmitted in the heart and lungs, and SARS-CoV-2 mainly attacks alveolar epithelial cells, leading to respiratory side effects [33]. Because ACE2 has a protective effect on the lungs, when healthy people are infected with SARS-CoV-2, membrane proteins bind to ACE2 in alveolar epithelial cells, allowing the infection to disrupt the ACE2 lung insurance pathway, leading to to viral pathogenesis (Figure 4) [31,32]. The Renin-Angiotensin System (RAS) may be a key factor controlling the cardiovascular system and water and electrolyte balance, influencing the body’s organs and capabilities [34]. Both ACE2 and Pro have positive effects on the RAS in controlling the steady state of the cardiovascular and renal systems as well as in managing extracellular fluid volume. There are two basic pathways: one is the classical RAS pathway, which is the ACE/AngII/AT1R pathway, and the central timing ACE2/ang1-7/Mas and ACE2/ang1-9/ AT2R, which regulate each other. The ACE2 movement leads to the promulgation of the AngII/AT1R hub and plays an important role in promoting improvement in cardiovascular diseases. This strongly suggests that ACE2 is an essential controller of cardiac activity in vivo [35,36]. SARS-CoV-2 uses ACE2 as a utility receptor to enter human cells. In this way, ACE2-related pathways may play a role in the cardiovascular damage of COVID-19 [37].
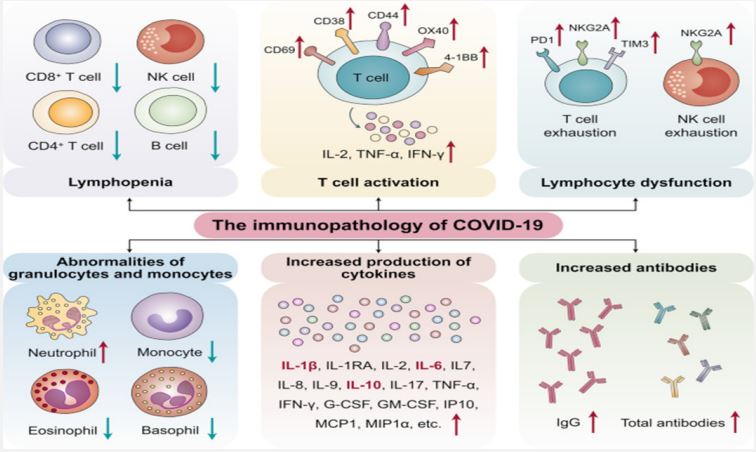
Immunopathological mechanisms of SARS-CoV-2 infection: The pathogenesis of COVID-19 isn’t characterized but reports from numerous nations show that the infection has the same instrument by which it enters or attacks have cells as SARSCOV. The origin of SARS-CoV-2 isn’t well-established, in any case, it is built up that bats are the source of related infections which human to human transmission plays a basic part in its pathogenesis [38-40]. After entering into target cells taking after Spike protein affiliation with its receptor [41], viral RNA is typified and polyadenylated, and encodes different auxiliary and non-structural polypeptide qualities. These polyproteins are cleaved by proteases that show chymotrypsin-like action [42]. In spite of the fact that transmembrane serine protease 2 (TMPRSS2) is the major protease related with CoV enactment and has been connected to SARS-CoV-2 actuation, later prove from single cell RNA-sequencing (scRNA-seq) examination appears that ACE2 and TMPRSS2 are not communicated within the same cell [43] proposing the association of other proteases such as cathepsin B and L in this handle. In common, design acknowledgment receptors (PRRs) recognize attacking pathogens counting infections [44]. Infections evoke a few key have resistant reactions such as expanding the discharge of incendiary variables, acceptance and development of Dendritic Cells (DCs) and expanding the blend of sort I Interferons (IFNs), which are critical in constraining viral spread [45]. Both the intrinsic and procured resistant reaction are actuated by SARS-CoV-2. CD4+ T cells invigorate B cells to deliver virus-specific antibodies counting Immunoglobulin (Ig)G and IgM and CD8+ T cells specifically slaughter virus-infected cells (Figure 5). T helper cells produce pro-inflammatory cytokines and mediators to help the other immune cells. SARS-CoV-2 can block the host immune defense by suppressing T cell functions by inducing their programmed cell death e.g. by apoptosis. Furthermore, the host production of complement factors such as C3a and C5a and antibodies are critical in combating the viral infection [46,47].
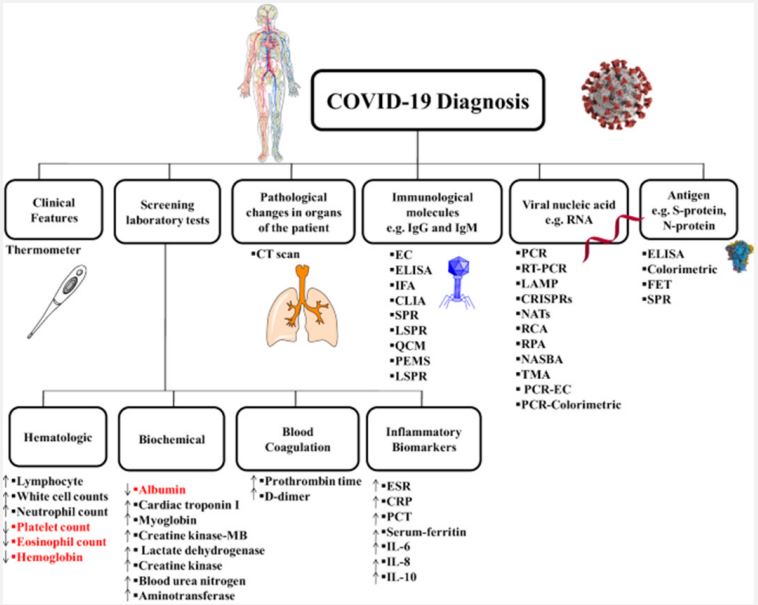
Diagnosis of COVID-19: Although the atomic and fundamental characteristics of SARS-CoV-2 were initially obscure, in a very short time research from laboratories and biomedical companies has examined key aspects of infection, helping analysts around the world by creating different demonstration devices to accurately identify COVID-19 [47,48]. Among these devices, the most commonly used and approved techniques are rapid antigen or antagonist tests, serum enzyme immunoassays, and RT-PCR-based atomic tests. Each of these three types of demonstration tests can be connected to one minute of precise contamination [49]. It should be noted that atomic units, reagents and tests approved by the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) and approved by the FDA and EMA may be used approved in the US and Europe for demonstration purposes. Although the enzyme immunoassay strategy (classical or rapid strategy) and the atomization strategy are the most widely used methods for diagnosing COVID-19, other methods were used early in the strategy translation to identify positive patients and experts on the cause of illness. Among these approaches, viral culture and Next-Generation Sequencing (NGS) strategies have proven to be crucial for recognizable evidence of the novel coronavirus and for characterization its atomic structure [50]. These two procedures have led to the complete characterization of the viral genome and structure of viral proteins, allowing an understanding of the tools of viral action, modes of transmission, clinical efficacy, and the advancement of rehabilitation techniques and symptomatic treatment equipment [51,52]. In addition to these conventional techniques, other demonstration strategies are being developed and validated or are currently finding application in investigative contexts. Among these strategies, computerized PCR, isothermal enhancement strategy, Clustered Interspaced Short Palindromic Repeats (CRISPR/ Cas), biosensor-based symptom strategy, microsatellite-based strategy microelectronics, etc., solve the arsenal for effective analysis of COVID-19 and effectively study the epidemiology of widespread infection [53,54]. Notably, clinical and radiological examinations have shown significant symptomatic selection, especially in the early stages of transmission, when approved atomic and serological tests were not yet available. Browser available (Figure 6) [54,55].
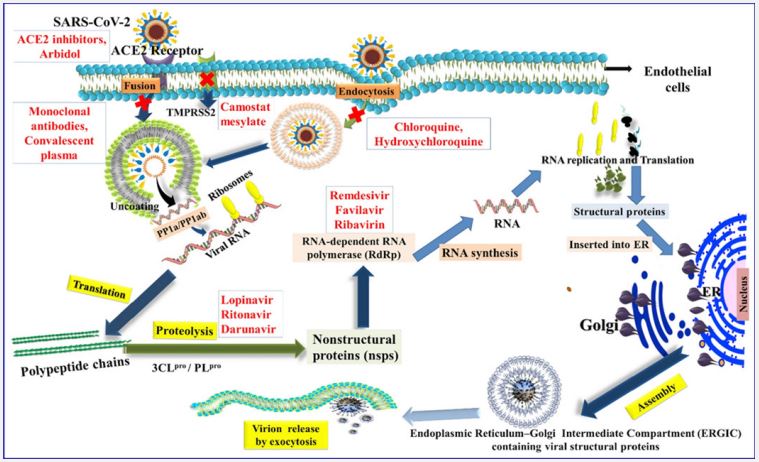
Therapeutic strategies: The atomic and fundamental properties of SARS-CoV-2 were initially obscure, but in a short time, research from laboratories and medical firms has examined key aspects of infection, aiding researchers around the globe. Creating various demonstration devices to demonstrate COVID19’s accuracy [47,48]. Rapid antigen or antagonist tests, serum enzyme immunoassays, and RT-PCR-based atomic tests are the most commonly used and approved techniques. One minute of precise contamination can be connected to each of these three types of demonstration tests. It is important to note that atomic units, reagents, and tests approved by the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) and approved by the FDA and EMA may be used. The approval was granted in the United States and Europe. It is for demonstration purposes only. The classic or rapid strategy for enzyme immunoassay and the atomization strategy are the most widely used methods for diagnosing COVID-19, but other strategies were employed at an earlier stage. Positive patients and experts on the cause can be identified through translation. The symptoms of illness. For recognizable evidence of the novel coronavirus, viral culture and next-generation sequencing strategies have been crucial. It has an atomic structure. The combination of these two approaches has resulted in the complete elucidation of the viral genome and the morphology of viral proteins, facilitating a comprehension of the methods of viral action, modes of dissemination, therapeutic efficacy, and the advancement of rehabilitation strategies and symptomatic treatment tools [52,51]. Besides the aforementioned conventional methods, various demonstration strategies are currently being developed and tested, or are already being employed in investigative settings. Computerized PCR, isothermal enhancement strategy, Clustered Interspaced Short Palindromic Repeats (CRISPR/Cas), biosensor-based symptom strategy, microsatellite-based strategy microelectronics, etc., are some of the strategies that solve the arsenal for efficient analysis of COVID-19. Studying the epidemiology of widespread infection is a valuable method for 19 and evaluating its effectiveness. Notably, diagnostic and radiological examinations have revealed significant symptomatic discrimination, particularly in the initial stages of transmission, when approved atomic and biological diagnostic procedures were still in development. The browser is available. There is a figure available (Figure 6).
The escalating number of COVID-19 infections around the globe has highlighted the timidity of the therapeutic team. A few other organs/organ frameworks may also be affected by viral infections-mediated inflammatory state and immonumodulation, apart from the lungs [57,58]. The abrupt onset of this viral infection has led to the confinement of individuals across nations. The day-to-day alteration in symptomatology and introduction has calmed the atomic investigate and formation sciences to come up with more secure and compelling helpful operators and immunizations. We are comprehending and doing combating this widespread with no proven therapeutics. The task at hand involves taking a comprehensive approach and providing exemplary assistance, in line with the apparent seriousness of the matter. While battling a widespread issue, it’s vital for health care specialists to stay up-to-date with the latest and develop helpful strategies for tackling the illness with greater viability. All the vital safety precautions must be taken to alleviate the auxiliary waves of the widespread. It is also imperative to optimize novel belief systems of cellular treatment conventions in aide to the advancement of antibodies, as these have the potential to demonstrate as the positive shades of a rainbow in the midst of the storm. It’s time to ask around on the definitive administration traditions by conducting randomized controlled trials, since security and effectiveness parameters need to be uncovered. Antibody planning tends to be a threephase (I, II, III) trial-based method (Table 2). The execution of the first test aims to check the era of resistance by selecting products from 10 (roughly 30-40) individuals [75,80]. A successful stage I trial continues to advance to stage II with the encouragement of hundreds of people. The aim is to distinguish the dose concentrations, immunogenicity, and security. The stage III trial will include the enrollment of countless individuals to assess the effectiveness of the vaccination in terms of its resistance response against the targeted malady disease. Many companies and universities are currently examining the improvement of an inoculation against SARS-CoV-2 with the Fusion for Plague Preparedness [76,70]. The information on the various immunizations that are supported and in progress is gathered here. The information provided by the producers indicates that this immunization is 95% secure. The viability of MV is 94% in line with the information provided by the producers and the U.S. Food and Drug Administration. In accordance with the two-dosage convention of SD/SD or LD/SD, the OAV was detailed to have a viability ranging from 62 to 90% After two measurements managed three weeks separated intramuscularly, the Sputnik-V Antibody had an adequacy of 91.6% in security.
Table 2: Different vaccines which can affect in COVID-19.
| Vaccines | Types * |
|---|---|
| Pfizer-BioNTech | mRNA [10,11] |
| Moderna | mRNA [12] |
| CVnCoV(or CureVac) | mRNA [13] |
| Oxford-AstraZeneca | Vector-ChAdOx [14] |
| Sputnik V by Gamaleya | Vector-Ad5 and Ad26 [15] |
| Johnson and Johnson | Vector-Ad26 [16] |
| Ad5-nCoV (or Convidecia) | Vector-Ad5 [17] |
| Sinopharm | Inactivated [18] |
| Sinopharm Wuhan | Inactivated [19] |
| CoronaVac | Inactivated [20] |
| Covaxin (or BBV 152) by BharatBiotech | Inactivated [21] |
| Novavax COVID-19 | Protein subunit [22] |
| EpiVacCorona by Vector Institute | Synthetic protein [23] |
| ZF 2001 | Protein-RBD dimer [24] |
*Virus-Like Particle (VLP) vaccines are not approved for usage yet.
Conclusion
The sudden growth of COVID-19 cases over the globe has raised concerns about the therapeutic society. Viral infectionsmediated inflammatory state and immonumodulation may have potentially unfavorable impacts on a few other organs/ organ frameworks, as well. The unforeseen onset of this viral contamination has led to the confinement of individuals across diverse nations. Changes in symptomatology and introduction have pacified the atomic investigate and formative sciences to come up with more secure and viable helpers. Currently, we comprehend and engage this widespread, despite the absence of demonstrated therapeutics [63,64]. The imperative is to take a holistic approach and provide steady support, in line with the apparent seriousness of the circumstance. While battling a widespread condition, it’s vital for health care specialists to keep themselves up to date with the most recent and advancing helpful techniques for tackling the condition with greater determination.
References
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020; 395: 470-473.
- Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): Current status and future perspectives. Int. J. Antimicrob. Agents. 2020; 55: 105951.
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med Res. 2020; 7: 1-10.
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020; 17: 259-260.
- Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr H. Treatment of SARS with human interferons. Lancet. 2003; 362: 293-294.
- Florindo HF, Kleiner R, Vaskovich-Koubi D, Acúrcio RC, Carreira B, Yeini E, Tiram G, Liubomirski Y, Satchi-Fainaro R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020; 15: 630-645.
- Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, Li PT, Dai J, Mok FK, Chen H, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J. Infect. 2013; 67: 606-616.
- Cheng KW, Cheng SC, Chen WY, Lin MH, Chuang SJ, Cheng IH, Sun CY, Chou CY. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antivir. Res.n. 2015; 115: 9-16.
- Wang Y, Sun Y, Wu A, Xu S, Pan R, Zeng C, Jin X, Ge X, Shi Z. Ahola T. et al. Coronavirus nsp10/nsp16 methyltransferase can be targeted by nsp10-derived peptide in vitro and in vivo to reduce replication and pathogenesis. J. Virol. 2015; 89: 8416-8427.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Nguyen-Van-Tam JS, Beck CR. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015; 211: 80-90.
- Graham RL, Donaldson EF, Baric RS. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013; 11: 836-848.
- DeWit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2020; 3: 2019-2208.
- Burrell CJ, Howard CR, Murphy FA. Fenner and White’s Medical Virology; Elsevier BV: Amsterdam, The Netherlands. 2017.
- Burrell AJ, Pellegrini B, Salimi F, Begum H, Broadley T, Campbell LT, Cheng AC, Cheung W, Cooper DJ, Earnest A, et al. Outcomes for patients with COVID-19 admitted to Australian intensive care units during the first four months of the pandemic. Med. J. Aust. 2021; 214: 23-30.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270-273.
- Hughes J, Wilson M, Luby S, Gurley E, Hossain M. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 2009; 49: 1743-1748.
- Kan B, Wang M, Jing H, Xu H, Jiang X, Yan M, Liang W, Zheng H; Wan K, Liu Q, et al. Molecular Evolution Analysis and Geographic Investigation of Severe Acute Respiratory Syndrome Coronavirus-Like Virus in Palm Civets at an AnimalMarket and on Farms. J. Virol. 2005; 79: 11892-11900.
- Zheng BJ, Guan Y, Wong KH, Zhou J, Wong KL, Young BWY, Lu LW, Lee SS. SARS-related Virus Predating SARS Outbreak, Hong Kong. Emerg. Infect. Dis. 2004; 10: 176-178.
- Shi Z, Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008; 133: 74-87.
- Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family Cluster of Middle East Respiratory Syndrome Coronavirus Infections. N. Engl. J. Med. 2013; 368: 2487-2494.
- Annan A, Baldwin HJ, Corman VM, Klose SM, Owusu M, Nkrumah EE, Badu EK, Anti P, Agbenyega O, Meyer B. Human betacorona virus 2c EMC/2012–related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013; 19: 456.
- Lau SKP, Li KSM, Tsang AKL, Lam CSF, Ahmed S, Chen H, Chan KH, Woo PCY, Yuen KY. Genetic Characterization of Betacoronavirus Lineage C Viruses in Bats Reveals Marked Sequence Divergence in the Spike Protein of Pipistrellus Bat Coronavirus HKU5 in Japanese Pipistrelle: Implications for the Origin of the Novel Middle East Respiratory Syndrome Coronavirus. J. Virol. 2013; 87; 8638-8650.
- Chan JFW, Kok KH, Zhu Z, Chu H, To KKW, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visitingWuhan. Emerg. Microbes Infect. 2020; 9: 221-236.
- Lu H, Stratton CW, Tang Y. Outbreak of pneumonia of unknown etiology in The mystery and the miracle. J. Med. Virol. 2020; 92: 401-402.
- Chan JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, Xing F, Liu J, Yip CCY, Poon RWS, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020; 395: 514-523.
- Fan Y, Zhao K, Shi ZL, Zhou P. Bat Coronaviruses. Viruses 2019; 11: 210.
- Mahase E, brazil: What do we know so far? BMJ. 2020; 368: 308.
- Hui DS; Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak J. Infect. Dis. 2020; 91: 264-266.
- Rodriguez-Morales, AJ, Bonilla-Aldana DK, Balbin-Ramon GJ, Rabaan AA, Sah R, Paniz-Mondolfi A, Pagliano, P.; Esposito, S. History is repeating itself: Probable zoonotic spillover as the cause of the 2019 novel coronavirus epidemic. Infez. Med. 2020, 28, 3–5.
- Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside ofWuhan, a: Retrospective case series. BMJ 2020; 368: m606.
- Ison MG, Hirsch HH. Community-acquired respiratory viruses in transplant patients: Diversity, impact, unmet clinicalneeds. Clin. Microbiol. Rev. 2019; 32: 00042-19.
- Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006; 6: 130.
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020; 104: 246-251.
- Hadjadj J. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020. https://doi.org/10.1126/science.abc6027.
- Li, Q. et al. Early transmission dynamics of novel coronavirusinfected pneumonia. N. Engl. J. Med. 2020; 1199-1207.
- Chen, G. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020; 2620-2629.
- Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus. Lancet. 2020; 497-506.
- Mehta P. et al. COVID-19: consider cytokine storm syndromes and immunosup- pression. Lancet. 2020; 1033-1034.
- Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020; 295: 4773-4779.
- deWit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2020; 117: 6771-6776.
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. 2020.
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G, et al. Remdesivir and chloroquine eectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30: 269-271.
- Han W, Quan B, Guo Y, Zhang J, Lu Y, Feng G, Wu Q, Fang F, Cheng L, Jiao N, et al. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J. Med. Virol. 2020; 92: 461-463.
- Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease. 2019. (COVID-19). Clin. Chem. Lab. Med. 2020.
- Lagier JC, Colson P, Tissot Dupont H, Salomon J, Doudier B, Aubry C, Gouriet F, Baron S, Dudouet P, Flores R, et al. Testing the repatriated for SARS-Cov2: Should laboratory-based quarantine replace traditional quarantine? Travel Med. Infect. Dis. 2020.
- Won J, Lee S, Park M, Kim TY, Park MG, Choi BY, Kim D, Chang H, Kim VN, Lee CJ, et al. Development of a laboratory-safe and low-cost detection protocol for SARS-CoV-2 of the coronavirus disease 2019 (COVID-19). Exp. Neurobiol. 2020.
- Loe elholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections–The state of the art. Emerg. Microbes Infect. 2020; 9: 747-756.
- World Health Organization Coronavirus Disease (COVID-19) Technical Guidance: Laboratory Testing for 2019-nCoV in Humans. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus- 2019/technical-guidance/laboratoryguidance. 2020.
- Phan,T. Novel coronavirus: From discovery to clinical diagnostics. Infect. Genet. Evol. 2020; 79.
- World Health Organization Novel Coronavirus (2019-nCoV), Situation Report 1. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019- ncov.pdf (accessed on 25 March 2020).
- Hsu LY, Chia PY, Lim JF. The Novel coronavirus (SARS-CoV-2) epidemic. Ann. Acad. Med. Singap. 2020; 49: 1-3.
- World Health Organization Coronavirus Disease 2019 Situation Report. Available online: https://www.who. int/docs/defaultsource/coronaviruse/situation-reports/20200326-sitrep-66-covid-19.pdf?sfvrsn=9e5b8b48_2 (accessed on 25March 2020).
- Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of covid-19–Studies needed. N. Engl. J. Med. 2020; 382: 1194-1196.
- Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown The mystery and the miracle. J. Med. Virol. 2020; 92: 401-402.
- Huang C, Wang Y, Li Z, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in. 2020; 395: 497-506.
- World Health Organization Director-General’s Opening Remarks at the Media Briefing on COVID-19–11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-directorgeneral-s-opening- remarks-at-the-media-briefing-on-covid-19--11-march. 2020.
- Perlman S, Netland J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009; 7: 439-450.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020; 395: 565-574.
- Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018.
- Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect. Dis Poverty. 2020; 9: 29.
- Centers for Disease Control and Prevention Novel Coronavirus. 2019. https://www.cdc.gov/coronavirus/2019-ncov/preventgetting-sick/prevention.html (accessed on 13 April 2020).
- World Health Organization Coronavirus Disease (COVID-19) Advice for the Public. Available online: https. 2020. //www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-forpublic (accessed on 30 March).
- World Health Organization. Available online. 2020. https://apps.who.int/iris/handle/10665/330987 (accessed on 30 March).
- Hopman J, Allegranzi B, Mehtar S. Managing COVID-19 in lowand middle-income countries. JAMA 2020. [CrossRef]
- Tran HN, Le GT, Nguyen DT, Juang RS, Rinklebe J, Bhatnagar A, Lima EC, Iqbal HMN, Sarmah AK, Chao, HP. SARS-CoV-2 coronavirus in water and wastewater: A critical review about presence and concern. Environ. Res. 2021; 193: 110265.
- Ding Z, Qian H, Xu B, Huang Y, Miao T, Yen HL, Xiao S, Cui L, Wu X, Shao W, et al. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 2021; 753: 141710. [CrossRef]
- Tang JW, Bahnfleth WP, Bluyssen PM, Buonanno G, Jimenez JL, Kurnitski J, Li Y, Miller S, Sekhar C, Morawska L, et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus (SARS-CoV-2). J.Hosp. Infect. 2021.
- SanJuan-Reyes S, Gómez-Oliván LM, Islas-Flores H. COVID-19 in the environment. Chemosphere. 2021; 263: 127973.
- Ni Y, Alu A, Lei H, Wang Y, Wu M, Wei X. Immunological perspectives on the pathogenesis, diagnosis, prevention and treatment of COVID-19. Mol. Biomed. 2021; 2: 1-26.
- Yoo JH. What We Do Know and Do Not Yet Know about COVID-19 Vaccines as of the Beginning of the Year 2021. J. Korean Med. Sci. 2021; 36: e54.
- Already Vaccinated? Here’s Why You Shouldn’t Stop Wearing Your Face Mask Yet-Cleveland Clinic-Health Essentials. Available 2021. online:https://health.clevelandclinic.org/already-vaccinated-heres-why-you-shouldnt-stop-wearing-your-face-maskyet/.
- Read JS. Reactogenicity, Contraindications, and Precautions: mRNA COVID19 Vaccines—Vermont Department of Health. 2021. Avail online:https://www.healthvermont.gov/sites/default/files/documents/pdf/COVID-19-HAN-AnaphlaxismRNACOVID-19Vaccines.pdf.
- Johnson & Johnson Prepares to Resume Phase 3 ENSEMBLE Trial of Its Janssen COVID-19 Vaccine Candidate in the U.S.—Johnson and Johnson Press Release. 2021. Available online: https://www.jnj.com/our-company/johnson-johnson-prepares-to-resume-phase- 3-ensemble-trial-of-its-janssen-covid-19-vaccinecandidate-in-the-us.
- Tan HX, Juno JA, Lee WS, Barber-Axthelm I, Kelly HG, Wragg KM, Esterbauer R, Amarasena T, Mordant FL, Subbarao K, et al. Immunogenicity of prime-boost protein subunit vaccine strategies against SARS-CoV-2 in mice and macaques.Nat. Commun. 2021; 12: 1-10.
- Wadman M. Novavax vaccine delivers 89% efficacy against COVID-19 in U.K.—But is less potent in South Africa. Science 2021.
- Jeon KY. COVID-19 Vaccines-Safety First, Alleged “Greater Good” Last. Am. J. Epidemiol. Public Health. 2020; 4: 12-16.
- McIntosh K. COVID-19: Epidemiology, Virology, and Prevention. UpToDate. 2021. Available online: https://www.uptodate.com/contents/covid-19-epidemiology-virology-and-prevention.
- Li YD, Chi WY, Su JH, Ferrall L, Hung CF, Wu TC. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020; 27: 104.
- Lee P, Kim CU, Seo SH. Kim D, J Current Status of COVID-19 Vaccine Development: Focusing on Antigen Design and Clinical Trials on Later Stages. Immune Netw. 2021; 21: 4.
- Forni G, Mantovani A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021; 28: 626-639.