Short Report
Volume 3, Issue 3
Electrocardiographic Variants of Ventricular Predominance in Arrhythmogenic Cardiomyopathy
Stefan Peters*
Clinic of Internal Medicine and Cardiology, UEK Norden, Germany.
Corresponding Author :
Stefan Peters
Tel: +49-4931-181-435;
Email: H.u.S.Peters@t-online.de
Received : Feb 26, 2024 Accepted : Mar 12, 2024 Published : Mar 19, 2024 Archived : www.meddiscoveries.org
Citation: Peters S. Electrocardiographic Variants of Ventricular Predominance in Arrhythmogenic Cardiomyopathy. Med Discoveries. 2024; 3(3): 1131.
Copyright: © 2024 Peters S. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Desmoglein-2 and Desmocollin-2 mutations cause different types of arrhythmogenic cardiomyopathy either dominant right, biventricular or dominant left forms. These two mutations present with myocardial cell necrosis and calcification [1,2]. Signs of electrocardiographic right ventricular hypertrophy can be found in more than 50% [3] besides typical ECG criteria of arrhythmogenic cardiomyopathy like right precordial epsilon waves and T-wave inversions [4], QRS fragmentation [5], localized right precordial QRS prolongation [6], terminal activation delay [7], and large Q-waves, small R-waves and T-wave inversion in lead aVR [8].
Case reports
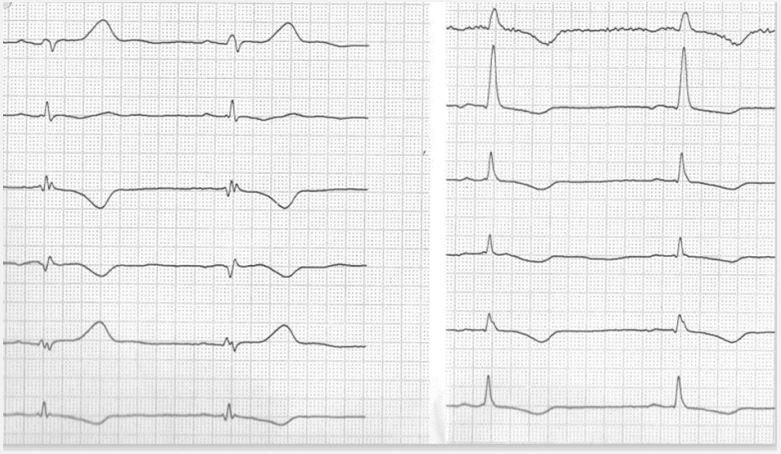
Case 1: A 42-old female patient presented with pain in the right leg. Venuous thrombosis was excluded. Two-dimensional echocardiography revealed the largy dilated right ventricle with reduced TASPE and localized dilatation of the RVOT and inferior sites of the right ventricle. Left ventricular function was normal with an ejection fraction of 65%. In the ECG a patient showed ECG features of right ventricular hypertrophy, right precordial T-wave inversions, typical features in lead a VR, localized right precordial QRS prolongation, and low voltage in limb leads. A asymptomatic form of arrhythmogenic cardiomyopathy was suspected. The case of this case in presented in Figure 1.
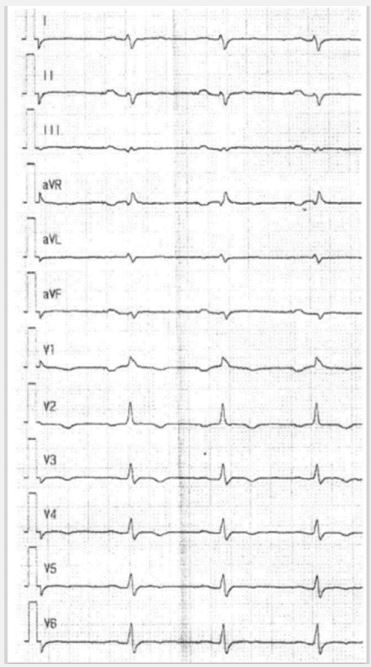
Case 2: A 38 year-old French male patient with biventricular heart failure grade IV was attended in hospital as a heart transplantation candidate. Echocardiography showed sincerely reduced left and right ventricular function. Biopsy revealed a biventricular form of arrhythmogenic cardiomyopathy. In the ECG signs of right ventricular hypertrophy, right precordial T-wave inversions, epsilon waves, and low voltage in limb leads were present. Genetics revealed a mutation in the desmoglein-2 gene. The ECG is shown in Figure 2.
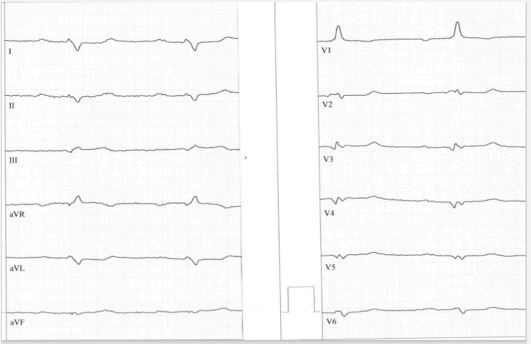
Case 3: A 33 year-old male patient was attended in hospital because of a heart failure grade III-IV in the university clinic in Heidelberg, Germany. Coronary artery disease was ruled out. Left ventricular angiography and cardiac MRI revealed the diagnosis of arrhythmogenic left ventricular cardiomyopathy with an ejection fraction of 28% and no abnormalities of the right ventricle. Despite higher dose of valsartan-sacubitril the ejection fraction was stable and an ICD was implanted. In the ECG signs of right ventricular hypertrophy, posterior fascicular block, significant Q waves in precordial leads, and low voltage im limb leads were present. The ECG of this case in presented in Figure 3.
Conclusion
Right dominant, biventricular and left dominant forms of arrhythmogenic cardiomyopathy are mostly present in lamin A/C, filamin C, TMEM 43, phospholamban, but also in desmoglein-2, desmocollin-2 mutations [9]. Signs of right ventricular hypertrophy at ECG due to myocardial cell hypertrophy and necrosis and calcification are by far mostly present in desmoglein-2 and desmocollin-2 mutations. In very rare cases plakoglobin cases of the isle of Naxos (petrified right ventricle) make the same ECG findings [10].
One of the presented cases genetics revealed positive desmoglein-2 mutation finding, but in the other cases desmoglein-2 or perhaps desmocollin-2 mutations are suspected.
In all cases twelve lead ECG revealed highly typical findings of the special type of arrhythmogenic cardiomyopathy and demonstrates the high predictive value of standard ECG. In all cases low voltage in limb leads is present, although case no. 1 represent a case of typical arrhythmogenic right ventricular cardiomyopathy without left ventricular involvement. It can be suspected, that ECG findings are the first sign of possible developing biventricular disease. In nearly 80% of cases right dominant arrhythmogenic cardiomyopathy reveal left ventricular abnormalities either by simple ECG or by imaging techniques [11].
Simple twelve lead ECG is a solid marker of all forms of arrhythmogenic cardiomyopathy and can predict in several cases the type of mutation findings [12].
References
- Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, Barnett P, et al. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med. 2009; 206: 1787-802.
- Peters S. Electrocardiographic signs of necrosis and calcification in arrhythmogenic cardiomyopathy. Ann Cardiol Vasc Med. 2023; 6: 1074.
- Peters S, Wittinger T. Electrocardiographic signs of right ventricular hypertrophy in desmoglein-2 and plakophilin-2 related arrhythmogenic cardiomyopathy. SL Clin Exp Cardiol. 2020; 3: 120.
- Zhang L, Liu L, Kowey PR, Fontaine GH. The electrocardiogaphic manifestations of arrhythmogenic right ventricular dysplasia. Curr Cardiol Rev. 2014;10: 237-45.
- Peters S. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia-cardiomyopathy. Heart Rhythm 200; 5: 1417-21.
- Peters S, Trümmel M, Koehler B, Westermann KU. The value of different electrocardiographic depolarization criteria in the diagnosis of varrhythmogenic right ventricular dysplasia/cardiomyopathy. J Electrocardiol. 2007; 40: 34-7.
- Cox MG, van den Smagt JJ, Wilde AA, Wiesfeld AC, Atsma DE, Nelen DE, et al. New ECG criteria in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electropfysiol. 2009; 2: 524-30.
- Peters S. Clinical importance of lead aVR in arrhythmogenic cardiomyopathy. Int J Cardiol. 2014; 176: 508-9.
- Graziano F, Zorzi A, Cipriani A, De Lazzari M, Buace B, Rigato I, et al. The 2020 Padua Criteria for diagnosis and phenotype characterization of arrhythmogenic cardiomyopathy in clinical practise. J Clin Med. 2022; 11: 279.
- Basso C, Tsatsopoulou A, Thiene G, Anastasakis A, Valente M, Protonotarios N. Circulation. 2001; 104: E132-3.
- Varrenti M, Preda A, Frontera A, Baroni M, Gigli L, Vargui S, et al. Arrhythmogenic cardiomyopathy; definition, classification and arrythmic risk stratification. J Clin Med. 2024; 13: 456.
- Peters S. Electrocardiographic differences in desmosomal and non-desmosomal arrhythmogenic cardiomyopathy. In J Cardiol. 2016; 203: 1005-6.