Short Commentary
Volume 3, Issue 3
Rationale for Living Donor Organ Transplantation
Angelika C Gruessner1; Rainer WG Gruessner2*
1Professor of Medicine, State University of New York, USA.
2Professor of Surgery, State University of New York, USA.
Corresponding Author :
Rainer WG Gruessner
Email: Rainer.gruessner@downstate.edu
Received : Feb 06, 2024 Accepted : Mar 12, 2024 Published : Mar 19, 2024 Archived : www.meddiscoveries.org
Citation: Gruessner AC, Gruessner RWG. Rationale for Living Donor Organ Transplantation. Med Discoveries. 2024; 3(3): 1130.
Copyright: © 2024 Gruessner RWG. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
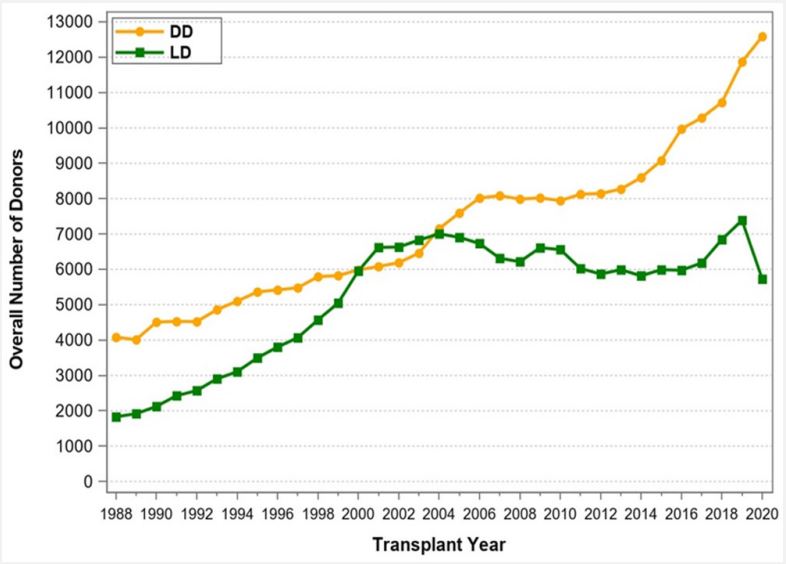
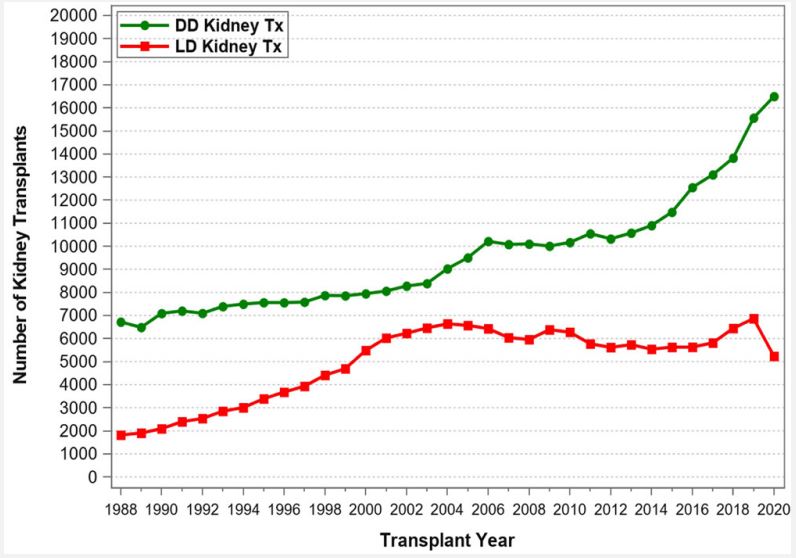
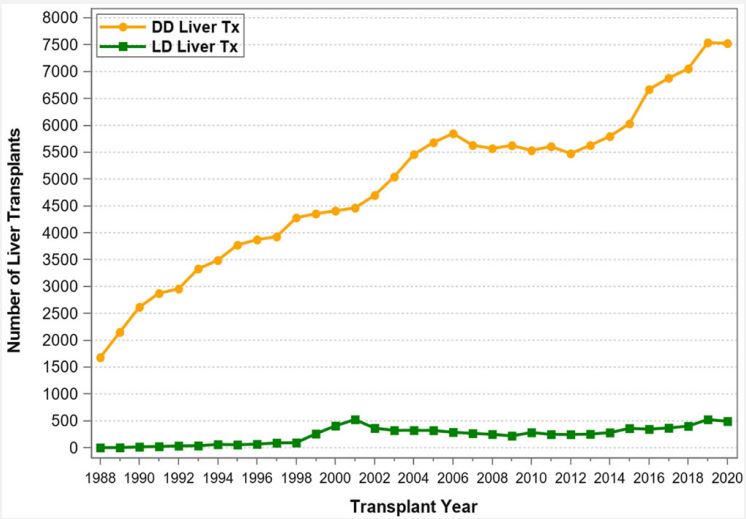
The use of Living Donors (LDs) for the purpose of solid organ transplantation has raised many questions and concerns from the very infancy of this field and have continued over time. These include ethical, medical, psychosocial, cultural, financial, racial, and other aspects. The obvious questions are: Why should organ donation from living donors be promoted and healthy individuals place their health and life at stake? Can we justify placing LDs in harm’s way? What is the status of living donation, and what are the risks for the donors? What safeguards and incentives should be implemented? Solid organ transplantation owes its very existence to LDs [1]. In the mid-1950s, a kidney transplant was immunologically successful only if the kidney was procured from a healthy twin donor: because of this specific relationship, the immunologic barrier that exists between Human Leukocyte Antigen (HLA) and nonidentical human beings could be overcome. With the advent of potent immunosuppressive therapy and with the introduction of mono- and polyclonal antibodies for induction and calcineurin inhibitors for maintenance therapy, transplants from Deceased Donors (DDs) became immunologically successful and routine. Nowadays, continued use of LDs is essential to transplant prospective candidates early and to avoid long waiting times associated with high morbidity and mortality rates. In fact, since the first successful kidney transplant (between identical twins) in 1954 by Joseph Murray [2], the number of LD transplants has continuously increased to about 7,000 per year until 2004, but then declined through 2008 (Figure 1). Beginning in 2016, the number of LD transplants started to increase again from about 6,000 to 7,500 transplants in 2019. In 2020, the COVID-19 pandemic had a significant impact on LD transplants and the number dropped to below 6,000 transplants. Notably, between 2001 and 2004 the number of living donors surpassed the number of deceased donors in the United States (Figure 1). The initiation of the “Donation and Transplantation Breakthrough Collaborative” (sponsored by the Department of Health and Human Services with key national leaders and practitioners from the transplantation and hospital communities) in April 2003 increased awareness of organ donation, in particular for DDs [3,4]. As a result, since 2004, the number of DDs has again surpassed the number of LDs (Figure 1). Another justification for increasing LD transplants is the ever-widening gap between patients waiting for a transplant and the availability of deceased donors. According to United Network for Organ Sharing (UNOS)/ Organ Procurement and Transplantation Network (OPTN), by the end of 2020, 91,099 patients were on the kidney transplant waiting list, but only 22,817 kidney transplants were performed; an additional 11,886 patients were on the liver transplant waiting list, but only 8,906 transplants were performed. Every day 17 patients died while waiting for an organ. Also, according to UNOS/OPTN, from January 1, 2016, through December 31, 2020, 178,059 organ transplants were performed in the United States (Table 1). Of all abdominal organ transplants, kidney transplants used the most LDs (Figure 2): a total of 29,983 LD kidney transplants took place during this 5-year period, representing 29.5% of all kidney transplants. The number of LD liver transplants has slightly increased to about 500 transplants per year in 2019 and 2020 (Figure 3); a total of 2,128 LD liver transplants took place during this 5-year time period, representing 5.6% of all liver transplants (Table 1). In contrast, there were no LD pancreas transplants and only one LD intestinal transplant performed during the 5-year time period from 2016 to 2020. Likewise, thoracic transplants from LDs are also only very rarely performed. Only 3 LD domino heart transplants, but no LD lung transplants were reported to UNOS during the 5-year time period. Of note, 21 LD uterus transplants were performed during the 5-year period; the uterus is the only organ that used more LDs than DDs (n=12). Given these numbers, it is evident that the use of LDs for kidney transplants has a significant impact on the treatment of end-stage renal disease in the United States—in 2020 alone, 213 out of 233 kidney transplant centers (91.4%) used LDs. The number of LDs used for liver transplants is the second highest of all solid organs. In 2020, 63 out of 142 liver transplant centers (44.4%) performed LD liver transplants. Pancreas and intestinal transplants have been performed rarely over the past decade and only few centers are able to perform these types of transplants. Of note, 2 out of 3 transplant centers that performed uterus transplants used LDs. The main reason for the high LD kidney transplant numbers is that the kidneys are paired organs, so kidney procurement does not require parenchymal dissection (as in liver or pancreas procurement) or reconstruction of organ integrity (as in intestinal procurement). The main reason for the unpaired liver to be considered much more frequently than the pancreas or the intestine for LD transplantation is the immediately livesaving nature of liver transplants. The use of an LD for a uterus transplant is completely different than for all other abdominal organs: it is based on the conscious decision of a woman to have no more pregnancies of her own. Another reason why LD kidney transplant numbers are so much higher than LD transplants of any other organ is this well documented fact: in kidney transplants the half-life of an LD (vs DD) graft is almost twice as long [5]. A comprehensive analysis of UNOS data has confirmed significantly better outcome for LD vs. DD kidney transplants and has also demonstrated best overall LD outcome for LD pediatric liver transplants [6]. One of the most important as pect of LD transplantation is donor mortality and morbidity. It is noteworthy to mention that of the 3 least commonly performed LD transplants, i.e., the pancreas, the intestine, and the uterus, not a single donor death has been reported. In LD kidney donation, the mortality risk for the donor is extremely low: between 2015 and 2019, only 2 early deaths for medical reasons (0-30 days post-transplant) out of over 30,000 LD kidney transplants were reported to UNOS/OPTN. Based on published numbers, the mortality risk for kidney LDs has been estimated to be 1 in 4,000 to 10,000 and less than 0.03%. In addition, the morbidity risks associated with kidney donation are low: the risk of major complications is less than 3% [7,8]. In contrast, for liver LDs, depending on the type of resection, the risk of death and major complications is considerably higher; the mortality risk is estimated to be 1 in 900 for lateral segmentectomy and 1 in 500 for lobectomy. To put it in better perspective, the risk of death for a liver LD is approximately 0.3% or about 10 times higher than the risk for a kidney LD [9,10]. Likewise, the risk of major donor complications is 6-10 times higher for liver vs kidney LDs.
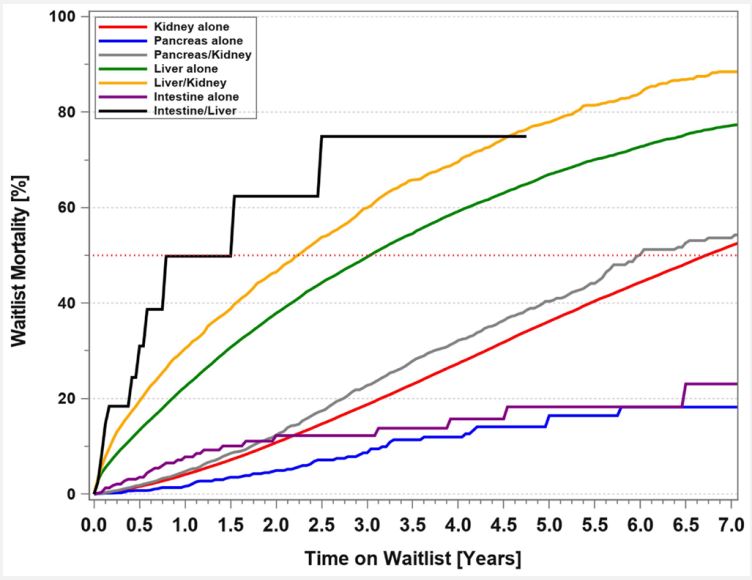
According to the WHO [11], the worldwide number of LD kidney transplants is over 550,000; LD liver transplants, about 69,600; LD pancreas transplants, about 200; and LD intestinal transplants, about 80. The first LD uterus transplant was performed in Sweden in 2012. A report about the worldwide experience lists 51 LD (vs. 11 DD) uterus transplants [12]. As of June 2022, about 100 uterus transplants have been performed worldwide and about 40 babies were born (personal communication, Liza Johannesson). As mentioned, in contrast to liver and kidney LDs, to date, no pancreas LD has died from procedurerelated causes. However, given the lack of regenerative capacity of insulin-producing cells (in contrast to the adaptive hypertrophy of a liver or kidney), the risk of developing diabetes mellitus post-donation and requiring oral antidiabetic medications or insulin administration is not insignificant [13,14]. For intestinal LDs to date, the mortality rate is also 0%; if the standardized surgical technique is used, the morbidity risk (e.g., diarrhea, vitamin malabsorption) is minimal [15]. Unfortunately, for all types of solid organ LDs, the exact short- and long-term complication rates are still unknown [16]. To better understand possible barriers to living donation and long-term risks attributable to donation, in the US, the Health Resources and Service Administration (HRSA) directed the Scientific Registry of Transplant Recipients (SRTR) in 2016 to establish a national registry of all living donor candidates and donors evaluated for at US transplant centers to acquire lifetime follow-up information [17,18]. The first results were published in 2021 [19]. But merely collecting LD data, whether through SRTR or, in Europe, through Eurotransplant, is not enough to protect the safety of LDs and to avoid organ trafficking. All countries that permit the use of LDs should be required to implement LD databases so that LD outcome can be compiled in a transparent and accountable fashion. Ideally, the World Health Organization (WHO) should oversee these databases to create a true international source for LD outcome data and for implementation of regulatory measures. The creation of an international LD database would also enable health professionals worldwide to accurately inform prospective LDs of their short and long-term risks [20,21]. Without doubt, the use of LDs significantly decreases transplant candidates’ mortality rates on the DD waiting list. For transplant candidates who were waitlisted between 1/1/2011 and 12/31/2020, the mortality rate while waiting for 3 years (5 years) for a solitary kidney transplant was 18.7% (36.2%) (Figure 4). For adult liver transplant candidates, the 3-year (5-year) mortality rate was 49.6% (67%), indicating that wider use of LDs could certainly decrease the high mortality rates. Not surprisingly, for abdominal organ transplants that have used the fewest number of LDs, the 3-year mortality rates on the DD waiting list have been lower: for solitary pancreas transplant candidates, 8.6%; for solitary intestinal transplant candidates, 12.2%. For transplant candidates who require both a kidney and a pancreas transplant, the mortality rates on the waiting list at 3 years (5 years) was 22.8% (40.4%). It was slightly higher than that for kidney-alone transplant candidates. The mortality rate has been highest for combined intestine–liver transplant candidates with 75.9% at 3 years. This is one of the reasons why dual LD organ transplants (LD pancreas-kidney transplants in adults and LD liver-intestinal transplants in children) have been successfully performed. It is apparent that increased use of living unrelated donors, of altruistic donors, and of donor exchange programs; for all types of transplants can further decrease DD waiting list mortality (Figure 4). The growing number of transplant candidates on the waiting list remains a huge concern-the proportional increase in the number of LD and DD transplants has not kept up with the proportional increase in listed candidates. As of December 31, 2020, a total of 85,229 candidates were still waiting for a solitary kidney transplant; 9,252, for a liver; 962, for a solitary pancreas; 3,368, for a combined pancreas and kidney; and 81, for an intestine (Table 2). This situation can only be ameliorated if society as a whole is educated about, and willing to support, nondirected or altruistic donation as a potential solution. One first step is to guarantee lifelong health insurance for all LDs. In addition, financial incentives for LDs may help bring about a steep increase in organ donation in the future. If financial incentives are considered, federal guidelines and laws as well as governmental oversight are required to avoid abuse of such a system.
In the United States, a more recent federal government initiative has focused on the need to increase the number of kidney transplants. In July of 2019, the U.S. Department of Health & Human Services (HHS) launched the ‘Advancing American Kidney Health’ Initiative. One of the three specific goals is to double “the number of kidneys available for transplant by 2030” [22]. An important factor to achieve this goal is a substantial increase in the number of kidney transplants from living donors. Executive Order 13879 of July 10, 2019, specifically states under Section 8: “Supporting Living Organ Donors. Within 90 days of the date of this order, the Secretary shall propose a regulation to remove financial barriers to living organ donation. The regulation should expand the definition of allowable costs that can be reimbursed under the Reimbursement of Travel and Subsistence Expenses Incurred Toward Living Organ Donation program, raise the limit on the income of donors eligible for reimbursement under the program, allow reimbursement for lost-wage expenses, and provide for reimbursement of childcare and elder-care expenses” [22].
There have also been U.S. state government initiatives such as New York state Senate Bill S1838 which proposes lifetime, premium-free insurance [provision] through the New York state of health marketplace to a person who donates a kidney during the course of his or her lifetime [and] establishes the kidney donor insurance fund [23]. Unfortunately, the implementation of federal and state reforms was slowed down by the impact of the 2020 COVID-19 pandemic. However, even with a possible increase in public willingness to donate for financial reasons, the major concern of such a program remains the same: the safety of LDs. Although we can certainly further minimize LD morbidity and mortality, we will not ever be able to completely eliminate the risks of donation. It takes courage and vision on the part of the LDs to accept these risks to end another human being’s suffering. For those reasons, this book is dedicated to all living donors.
Table 1: Number and type of transplants performed in the US between 1/1/2016 and 12/31/2020.
| Type of Transplant | Total Number of DD Transplants |
Number of LD Transplants |
|---|---|---|
| Kidney transplants alone | 71,539 | 29,983 |
| Kidney/Pancreas transplants | 4,094 | 0 |
| Solitary Pancreas transplants | 630 | 0 |
| Liver transplants alone | 35,667 | 2,128 |
| Liver/Kidney transplants | 3,649 | 0 |
| Intestinal transplants | 226 | 1 |
| Heart transplants | 15,822 | 3 |
| Heart/Kidney transplants | 1,040 | 0 |
| Heart/Lung transplants | 175 | 0 |
| Lung transplants | 12,447 | 0 |
| Multi – organ transplants | 622 | 0 |
| Uterus | 12 | 21 |
| Total number | 145,923 | 32,136 |
Table 2: UNOS/OPTN US number of patient’s registration for most frequent organ transplants between 1/1/2000 as of 12/31/2020 and number of patients still waiting at 12/31/2020.
| Type of Transplant | Total Number of DD Transplants |
Total Number of Registrations |
|---|---|---|
| Kidney transplants alone | 71,539 | 627,267 |
| Kidney/Pancreas Transplants | 4,094 | 41,950 |
| Solitary Pancreas transplants | 630 | 10,261 |
| Liver transplants alone | 35,667 | 200,593 |
| Liver/Kidney transplants | 3,649 | 17,282 |
| Liver/Intestine | 226 | 713 |
| Intestinal transplants | 15,822 | 1,296 |
| Heart transplants | 1,040 | 73,428 |
| Heart/Kidney transplants | 175 | 3,985 |
| Heart/Lung transplants | 12,447 | 1,326 |
| Lung transplants | 622 | 48,909 |
Disclaimer
The data reported herein have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
References
- Gruessner RWG, Gruessner AC. Rationale for Living Donor Organ Transplantation. In: Living Donor Organ Transplantation. 2nd Edition. Editors: Gruessner RWG, Benedetti E. Elsevier Inc, San Diego. 2024; 1: 3-8.
- Murray JE, JP Merril, and JH Harrison, Renal homotransplantation in identical twins. Surg Forum. 1955; 6: 432-436.
- Shafer TJ, et al. US organ donation breakthrough collaborative increases organ donation. Crit Care Nurs Q. 2008; 31(3): 190-210.
- Organ Donation Breakthrough Collaborative, accessed. 2022. Available from: http://www.ihi.org/resources/Pages/ImprovementStories/OrganDonationBreakthroughCollaborative.aspx.
- Kwong AJ, et al. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant. 2021; 2: 208-315.
- Gruessner RWG and AC Gruessner, Solid-organ TransplantsFrom Living Donors: Cumulative United States Experience on 140,156 Living Donor Transplants Over 28 Years. Transplant Proc. 2018; 50(10): 3025-3035.
- Gaston RS, V Kumar and AJ Matas, Reassessing medical risk in living kidney donors. J Am Soc Nephrol. 2015; 26(5): 1017-9.
- Lentine KL, NN Lam and DL Segev, Risks of Living Kidney Donation: Current State of Knowledge on Outcomes Important to Donors. Clin J Am Soc Nephrol. 2019; 14(4): 597-608.
- Kim PT and G Testa, Living donor liver transplantation in the USA. Hepatobiliary Surg Nutr. 2016; 5(2): p. 133-40.
- Hong, SK, et al., Long-Term Outcomes after Living Liver Donation: Analysis of National Data Base. Transplantation. 2018. 102: 20.
- Global Observatory on Donation and Transplantation. 3/29/2022]; Available from: www.transplant-observatory.org. 2022.
- Brannstrom M, MA Belfort and JM Ayoubi, Uterus transplantation worldwide: clinical activities and outcomes. Curr Opin Organ Transplant. 2021; 26(6): 616-626.
- Gruessner RW, et al. Pancreas transplants from living donors: short- and long-term outcome. Transplant Proc, 2001; 33(1-2): 819-20.
- Kirchner VA, et al. Long-term Outcomes for Living Pancreas Donors in the Modern Era. Transplantation. 2016; 100(6): 1322-8.
- Gruessner RW and HL Sharp, Living-related intestinal transplantation: first report of a standardized surgical technique. Transplantation. 1997; 64(11): 1605-7.
- Mulligan DC, A worldwide database for living donor liver transplantation is long overdue. Liver Transpl. 2006; 12(10): 1443-4.
- Kasiske BL, et al. OPTN/SRTR 2020 Annual Data Report: Living Donor. Am J Transplant. 2022; 2: 553-586.
- Kasiske BL, et al. The Living Donor Collective: A Scientific Registry for Living Donors. Am J Transplant. 2017. 17(12): 3040-3048.
- Kasiske BL, et al. Outcomes of Living Kidney Donor Candidate Evaluations in the Living Donor Collective Pilot Registry. Transplant Direct. 2021; 7(5): 689.
- Kiberd BA, Estimating the longterm impact of kidney donation on life expectancy and end stage renal disease. Transplant Res. 2013; 2(1): 2.
- Lentine KL and A Patel, Risks and outcomes of living donation. Adv Chronic Kidney Dis. 2012; 19(4): 220-8.
- Advancing American Kidney Health. 2019 3/28/2022]; Available from: https://www.federalregister.gov/documents/2019/07/15/2019-15159/advancing-american-kidneyhealth.
- Senate Bill S1838. 3/29/2022]; Available from: https://www.nysenate.gov/legislation/bills/2021/S1838. 2022.