Research Article
Volume 3, Issue 2
Targeted Screening for Undiagnosed Chronic Kidney Disease among Asymptomatic Immediate Family Members of Chronic Kidney Disease Patients: Experience from a Teaching Hospital in South Eastern Nigeria
Bartholomew C Ozuemba1 ; Chidiebele M Ezeude2*
1Department of Internal Medicine, Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria.
2Department of Internal Medicine, Faculty of Medicine, Nnamdi Azikiwe University, Awka, Nigeria.
Corresponding Author :
Chidiebele Malachy Ezeude
Email: cm.ezeude@unizik.edu.ng & docchidi@yahoo.co.uk
Received : Jan 26, 2024 Accepted : Feb 21, 2024 Published : Feb 28, 2024 Archived : www.meddiscoveries.org
Citation: Ozuemba BC, Ezeude CM. Targeted Screening for Undiagnosed Chronic Kidney Disease among Asymptomatic Immediate Family Members of Chronic Kidney Disease Patients: Experience from a Teaching Hospital in South Eastern Nigeria. Med Discoveries. 2024; 3(2): 1125.
Copyright: © 2024 Ezeude CM. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Chronic Kidney Disease (CKD) is a worldwide epidemic that is associated with a huge morbidity, mortality and high healthcare cost. In Nigeria, the number of patients with CKD progressing to End Stage Renal Disease (ESRD) is increasing. Early screening of individuals who are at risk of CKD has been advocated by several studies because it would reduce the morbidity and mortality associated with late presentation. Studies have found that the immediate family members/First-Degree Relatives (FDRs) of CKD patients have a greater prevalence of CKD compared with the general population and hence should be screened for kidney disease at any available opportunity.
There is paucity of data on the prevalence of CKD in FDRs of CKD patients in the sub-Saharan Africa generally. This study is aimed at adding to the current limited data on this important topic.
Methodology: This was a case-controlled prospective study of 300 subjects: 150 FDRs of CKD patients and the same number of sex and age-matched control subjects carried out at Nnamdi Azikiwe University Teaching Hospital, Nnewi, South Eastern, Nigeria.
The study and control subjects were screened for CKD using urine Albumin Creatinine Ratio (uACR) and Estimated Glomerular Filtration Rate (eGFR). These were repeated after three months for FDRs and controls with initial abnormal results. CKD was defined as eGFR<60 ml/min/1.73 m2 and/ or albuminuria >3 months.
Results: A significant high prevalence of CKD was found in the FDRs of CKD patients compared with the controls [26.7% versus 9.3%; p<0.001; OR (95% CI)=3.5(1.8-6.8)].
Conclusion: The prevalence rate of CKD was high among FDRs of CKD patients. Screening for CKD among FDRs of CKD patients would identify early disease and thus facilitate appropriate intervention that would retard disease progression and reduce the increasing global burden of CKD.
Keywords: Asymptomatic; Chronic kidney disease; Immediate family members; Undiagnosed.
Introduction
Chronic Kidney Disease (CKD) can be defined as abnormalities of kidney structure or function, present for >3 months with implications for health or Glomerular Filtration Rate (GFR)<60 ml/min/1.73 m2 for >3months [1].
Chronic Kidney Disease (CKD) constitutes a major public health problem in both the developing and the developed countries [2]. The economic impact of CKD on the healthcare and family is enormous [2]. This is due to its poor outcome and affectation of the individual’s quality of life and productivity [2]. In sub-Saharan Africa, including Nigeria, CKD is known to affect people aged between 20 and 50 years and these fall within the very productive years [3]. Furthermore, End-Stage Renal Disease (ESRD) occurs at an earlier age among people of African descent when compared with 63 years observed in other ethnic groups [3]. These facts underscore the importance of early CKD detection and prevention especially in the resource-poor sub Saharan African setting [3]. However, majority of people with CKD remain undetected, partly due to the asymptomatic nature of the disease at the early stages [3].
The prevalence rate of CKD worldwide is about 8 to 16% [2]. In the United States, the prevalence rate of CKD was found to have increased from 2.7% to 9.2% between the years 2000 and 2010 [4]. In Africa, the overall prevalence was estimated to be 15.8% [5]. In Nigeria, there were more than 10 million cases of CKD [6]. In some parts of Nigeria, the prevalence of CKD was shown to be 11.4% [7] and 10.4% respectively [8].
The natural history of established CKD is its progression to ESRD or death, if no medical treatment is given [3]. The rate of CKD progression is determined by the underlying aetiology of CKD, associated co-morbidities and availability of treatment options [3]. The burden of CKD as already shown is enormous [9]. It is a strong predictor of cardiovascular disease and is associated with increased morbidity and mortality [10].
Notably, there are three to nine folds of greater risk of developing ESRD in people with family history of ESRD and this has added credence to genetic contribution in CKD pathogenesis [11,12].
Studies have shown that genetic component may underlie susceptibility to progressive renal disease [13]. The rate of CKD progression and development of ESRD is determined by genetic factors [14]. APOL1 mutations are also responsible for the increased risk of CKD in individuals of African descent[15]. APOL1 belongs to APOL gene family and consists of six genes on human chromosome 22 [16]. The risk of kidney disease conferred by APOL1 gene variants (G1 and G2) follow a recessive inheritance pattern [17]. About 13% of African Americans carries these high risk genotypes while 50% carries at least one risk allele [16]. In sub-Saharan Africa, about 5% to 50% of the population has at least one risk allele [16].
Chronic kidney disease can be detected via routine screening with serum chemistry profile and urine studies [18]. Patients with CKD may less commonly present with symptoms such as foamy urine, nocturia, flank pain or decreased urine output [18]. In advanced CKD, patients may present with fatigue, poor appetite, nausea, vomiting, metallic taste, unintentional weight loss, and changes in mental state, difficulty in breathing or peripheral oedema [18]. Additional symptoms that may suggest a systemic cause (such as haemoptysis, rash, lymphadenopathy, hearing loss) or urinary tract obstruction (such as hesitancy, urgency, frequency, or incomplete bladder emptying) should be explored [18].
The prevalence of CKD among the immediate family members of CKD patients, also referred to as the First-Degree Relatives (FDRs) of CKD patients in a Nigerian study was 37.4% [19]. A key public health strategy in CKD prevention and management is screening of high risk populations such as people with family history of CKD. Several studies have shown that kidney disease is prevalent in first degree relatives (parents, siblings and children) of CKD subjects, especially in Africans and African Americans [15,21,22]. In developing countries like Nigeria, there is shortage of dialysis and renal transplant facilities which are the treatment options for ESRD. Therefore, early detection, prevention and treatment of CKD in FDRs of CKD patients are essential in decreasing the economic and personal burdens of CKD in Nigeria and will delay the progression to advanced stages. FDRs of CKD patients are easily approachable, as they often accompany their relatives to hospital. However, there is paucity of data on the prevalence rate of CKD among FDRs of CKD patients in Nigeria. This study aimed at answering this research question: is there a difference between the prevalence of CKD in FDRs of CKD patients compared with the healthy FDRs of their non CKD counterparts at Nnamdi Azikiwe University Teaching Hospital, Nnewi in South Eastern Nigeria?.
Materials and methods
Study design
This was a prospective study conducted among consenting adult first degree relatives of patients with CKD at Nnamdi Azikiwe University Teaching Hospital (NAUTH) Nnewi, South Eastern Nigeria.
The study was conducted from September 2018 to August 2019 in the Nephrology Outpatient Clinic and Haemodialysis unit of NAUTH, Nnewi.
NAUTH is a tertiary hospital domiciled in Nnewi, Anambra State that also serves the neighbouring states of Imo, Abia, Enugu and Delta.
The Nephrology clinic cares for Nephrology and hypertensive cases, runs every Tuesday and has an average turnover of about fifty patients per week. Maintenance haemodialysis operates morning, afternoon and night sessions every day of the week.
Study population
The participants for the study comprised two groups. The first group consists of the subjects who were the FDRs (parents, siblings and children) of CKD patients attending NAUTH Nephrology clinic, aged 18 years and above.
The second group consisted of an age and sex matched control group recruited from the General Outpatient Department (GOPD) of NAUTH, hospital staff and medical and paramedical students.
Consecutive recruitment of consenting 54 CKD patients (with eGFR <60 ml/min/1.73 m2 ) was done using a simple random sampling. A pool of the names of the FDRs of CKD patients was made and a possible three selected by balloting giving a total of 162 study subjects. In families with less than four FDRs, all the members were selected. FDRs of CKD patients that did not consent to the study were replaced from the pool. The selected FDRs were invited for the study by their relatives (CKD patients). 162 FDRs commenced the study, while 12 were lost to follow-up and 150 completed the study and their data analyzed. The study purpose and benefits of early CKD detection among their family members were explained to the CKD patients and their FDRs. For the CKD patients, information on the diagnosis of their CKD and presumed aetiology was obtained from the hospital records.
For the controls, 152 subjects were selected from GOPD, hospital staff and students of NAUTH, Nnewi. Of these, 150 controls completed the study, while 2 were excluded on account of positive retroviral screening results. The 150 controls that completed the study were individually matched with each study subject for age (±2 years) and gender (exact).
One hundred and fifty FDRs of CKD patients and 150 controls completed the study. All the study participants had two contacts with the researcher during two separate clinic visits: the baseline visit and follow-up visit. The first contact involved filling of study questionnaire, anthropometric measurements, blood pressure measurement, and blood and urine samples collection for laboratory analysis.
The second contact, three (3) months later and was for the study and control subjects whose estimated GFR came out to be <60 ml/min/1.73 m2 and/or urine ACR<30 mg/g at baseline visit. Follow up laboratory tests done at this meeting were serum creatinine and urine ACR.
A structured, self designed questionnaire was used to extract the socio-demographic and other relevant clinical data.
The inclusion criteria for all the study participants were age of 18 years and above and provision of a formal written informed consent.
The study subjects were excluded from the study if they had febrile illness, urinary tract infection, heart failure, retroviral disease or were pregnant.
The control subjects were excluded from the study if they had personal history of CKD, family history of CKD, febrile illness, urinary tract infection, heart failure, retroviral disease or were pregnant.
Ethical approval was obtained from the ethics committee of NAUTH, Nnewi before the commencement of the study.
Height and weight were measured with a stadiometer, waist circumference with a measuring tape and blood pressure with an Accoson mercury sphygmomanometer (Dekamet, England).
5 mls of blood that was collected from each of the study subjects and controls, was dispensed into a sterile plain container and allowed to clot and was retracted. The blood was centrifuged at 3000 rpm for 10 minutes and the serum separated into two aliquots and stored at -20°C. The analysis of all the biochemical parameters was done within one month of collection. The biochemical parameters analyzed included serum creatinine and retroviral screening.
The samples were analyzed at the laboratory of Nnamdi Azikiwe University Teaching Hospital, Nnewi. The serum creatinine was determined using Jaffe method [23]. Urine samples were collected between 7 am and 10 am in the morning. Urinalysis was done using Combi 10 dipsticks checking for proteinuria and infection. Positive nitrite or leucocyte test indicated presence of urinary tract infection. Retroviral screening was done using Determine kit (Alere DetermineTM HIV- 1/2, LOT 87029K100A, Japan).
Urinary albumin was estimated using turbidimetric immunoassay method (Lot No 30030164, AGAPEpeagent, Switzerland). Urinary creatinine was measured using Jaffe method [23].
Urinary albumin-creatinine ratio was calculated in milligram of albumin per gram of creatinine and results were interpreted as follows: Less than 30 mg/g was regarded as normal, between 30-300 mg/g was regarded as moderately increased and greater than 300 mg/g was regarded as severely increased [1].
The Estimated Glomerular Filtration Rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula. This is available online at: www.kidney.org/kls/professionals/gfr/calculator.cfm.
The equation is stated below:
186 x (serum creatinine in mg/dl) - 1.154 x (age in years) x 0.742 (for female) x 1.21 (for blacks) [24].
Data analysis
Data was analyzed using Statistical Package for Social Sciences (SPSS) version 21.0 (IBM Software Group, 200W. Madison St., Chicago, IL; 60606 USA). Categorical data was presented as frequency and percentage. The prevalence of CKD in FDRs of CKD patients and controls was reported as frequency and percentage. Differences in variables between the two groups (FDRs and control subjects) were statistically compared using MannWhitney U test for continuous variables that were not normally distributed and Chi square tests for categorical variables.
Definition of operational terms
Chronic kidney disease was defined as estimated GFR<60 ml/min/1.73 m2 for >3 months with or without evidence of kidney damage or if there was an indicator of kidney damage like albuminuria-in this study albuminuria was defined as urine Albumin Creatinine Ratio (ACR)≥30 mg/g for >3 months [1].
Results
A total of 300 subjects completed the study and their data were analyzed: 150 subjects (FDRs) and 150 controls.
Subjects characteristics
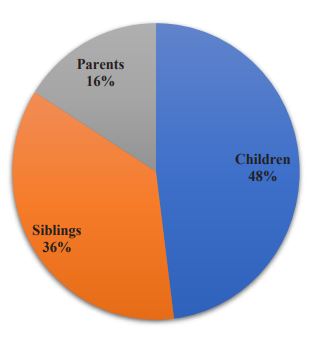
A total of 72 (48%) FDRs were children of CKD patients, 54(36%) were siblings of CKD subjects while 24(16%) were parents of the CKD patients (details in Figure 1).
Comparison of the socio-demographic characteristics of the FDRs and the controls
The mean age of the subjects and the control was 36 years. 46.0% of the subjects and the controls were males while 54.0% were females respectively. 52.4% of the FDRs and 63.3% of controls had tertiary education. 4.0% of FDRs and 0.7% of controls had no formal education. 72.7% and 74.0% of the subjects and controls were employed respectively while 26.7% and 25.3% of the FDRs and controls were students (details in Table 1).
Table 1: Socio-demographic characteristics of the First Degree Relative (FDRs) and the control group.
| Variables | FDR (%) N=150 |
Controls (%) N=150 |
Test Stat | p-value | |
|---|---|---|---|---|---|
| Median age (IQR) | 36.0(24.0) | 36.0(24.0) | U=11228.50 | 0.977 | |
| Gender | Male | 69(46.0) | 69(46.0) | χ2=0.000 | 1.000 |
| Female | 81(54.0) | 81(54.0) | |||
|
Level of Formal Education |
No formal | 6(4.0) | 1(0.7) | FT= 7.477 | 0.057 |
| Primary | 16(10.7) | 18(12.0) | |||
| Secondary | 50(33.3) | 36(24.0) | |||
| Tertiary | 78(52.0) | 95(63.3) | |||
|
Occupation- al status |
Employed | 109(72.7) | 111(74.0) | FT= 0.317 | 0.947 |
| Unemployed | 1(0.7) | 1(0.7) | |||
| Student | 40(26.7) | 38(25.3) | |||
|
Monthly income (Naira) |
<N20,000 | 47(31.3) | 50(33.3) | χ2= 0.137 | 0.403 |
| ≥N20,000 | 103 (68.7) | 100(66.7) | |||
IQR: Inter-Quartile Range; U: Mann-Whitney U test used; χ2: Chi square; FT: Stands for Fisher’s Exact used.
Comparison of the laboratory characteristics of the FDRs and controls
Urinary ACR was significantly higher in FDRs (28.93%) compared with the controls (14.68%) (P<0.001). Similarly median creatinine and median eGFR were significantly higher among FDRs (32.6% and 38.03%) compared to the controls (27.28% and 34.35%) respectively (P=0.009 and P=0.008) (details in Table 2).
Table 2: Comparison of the laboratory characteristics of the FDRs and controls at baseline.
| Variables | FDR (%) N=150 |
Controls (%) N=150 |
Test Stat | p-value |
|---|---|---|---|---|
| Median uACR (IQR) (mg/g) | 14.35(28.93) | 7.10(14.68) | U=5494.0 | <0.001* |
|
Median serum creatinine (IQR) (μmol/l) Median eGFR (IQR) (ml/min) |
84.00(32.60) 81.50 (38.03) |
80.40(27.28) 86.35(34.35) |
U=9300.5 U=9268.0 |
0.009* 0.008 |
IQR: Inter-Quartile Range; U: Mann-Whitney U test used; χ2 : Chi square; uACR: urine Albumin Creatinine Ratio; eGFR: estimated Glomerular Fil- tration Rate; TC: Total Cholesterol; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; TG: Triglycerides; *: statistically significant.
Prevalence of CKD (at baseline and after 3 months) among the FDRs
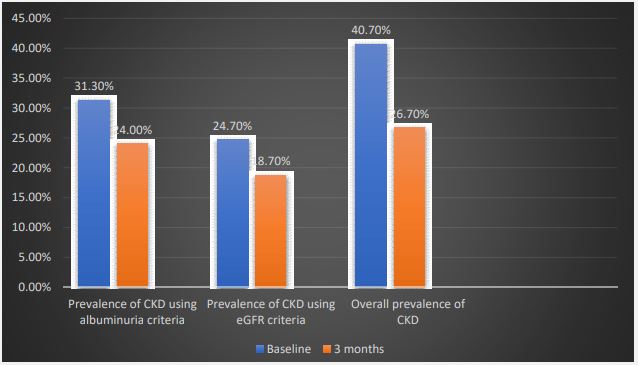
The prevalence of albuminuria among the FDRs reduced from 31.3% at initial test to 24.0% at third month. Also, there was a reduction in the prevalence of reduced eGFR (<60 ml/ min/1.73 m2 ) at baseline and after 3 months (24.7% versus 18.7% respectively) (Figure 2).
Prevalence of CKD (at baseline and after 3 months) among the controls
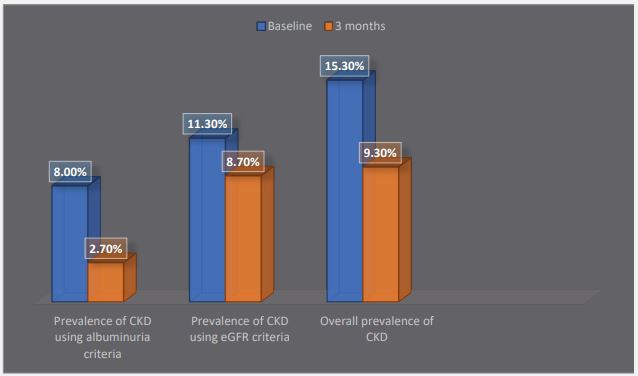
The prevalence of albuminuria among the controls reduced from 8% at initial test to 2.7% after 3 months. Also, there was a reduction in the prevalence of decreased eGFR (<60 ml/ min/1.73 m2 ) at baseline and after 3 months (11.3% versus 8.7% respectively) (Figure 3).
The prevalence of CKD among the controls was 15.3% at baseline and 9.3% after 3 months (Figure 3).
Table 3: Comparison of CKD screening test results among the participants after 3 months.
| Variables | Participants | otal (%) | Test Stat | p-value | |||
|---|---|---|---|---|---|---|---|
| FDRs (%) | Control (%) | OR (95% CI) | |||||
|
Median uACR (IQR) (mg/g) Albuminuria |
14.35(18.55) | 7.1(13.63) | 300(100.0) | U=5476.0 | <0.001* | ||
| Absent | 114(76.0) | 146(97.3) | 260(86.7) | 29.538 | <0.001* | - | |
| Present | 36(24.0) | 4(2.7) | 40(13.3) | 11.5(3.9-33.3) | |||
| Total | 150(100.0) | 150 (100.0) | 300(100.0) | ||||
|
Median serum creatinine
(IQR) (mg/g) Reduced eGFR |
84.00(32.25) | 80.4(27.28) | 300(100.0) | U=9357.0 | 0.012* | ||
| Absent | 122(81.3) | 137(91.3) | 259 (86.3) | 6.537 | 0.012* | - | |
| Present | 28(18.7) | 13(8.7) | 41(13.7) | 2.4(1.2-4.9) | |||
| Total | 150(100.0) | 150(100.0) | 300(100.0) | ||||
| Median eGFR in ml/min | 81.50(35.48) | 86.35(34.45) | 300(100.0) | U=9313.0 | 0.010* | ||
| CKD | |||||||
| Absent | 110(73.3) | 136(90.7) | 246(82.0) | 15.266 | <0.001* | - | |
| Present | 40(26.7) | 14(9.3) | 54(18.0) | 3.5(1.8-6.8) | |||
| Total | 150(100.0) | 150(100.0) | 300(100.0) | ||||
uACR: urine Albumin Creatinine Ratio; eGFR: estimated Glomerular Filtration Rate; IQR: Inter Quartile Range; χ2 : Chisquare; U: Mann-Whitney U test; *: statistically significant; OR: crude Odd Ratio; CI: Confidence Interval.
Comparison of CKD screening test results among the FDRs and controls after 3 months
The prevalence of CKD was significantly higher in FDRs when compared with the control group using the both criteria (Albuminuria & eGFR). The overall prevalence of CKD in FDR and control was 26.7% and 9.3% respectively (p<0.001). The FDRs were about 4 times more likely than the controls to have CKD [OR (95% CI)=3.5 (1.8-6.8)] (Table 3). The crude odd ratio of 3.5 was gotten using an online free odds ratio calculator.
Discussion
Background to the study
Chronic kidney disease is a worldwide epidemic that is associated with a huge morbidity, mortality and high healthcare cost. The number of patients with CKD progressing to ESRD is increasing [25]. Proper identification of individuals at risk of CKD will promote adequate primary, secondary and tertiary preventive measures that will help in reducing the burden of CKD in Nigeria.
Family history of CKD is a non-modifiable risk factor for kidney disease. There is paucity of data on the prevalence of CKD in FDRs of CKD patients in Nigerian literature. This study aimed at adding to the current knowledge on the prevalence of CKD in FDRs of CKD patients in Nigeria. This study in addition underscored the need for an appropriate counseling and referral of FDRs with detected kidney disease to nephrologists for expert care. The findings from this work supported the familial clustering of CKD.
Socio-demographic characteristics of FDRs of CKD patients
A higher proportion of females (54%) compared with males (46%) were recruited into this study. This observation was comparable to the study subjects recruited by Wei et al. and Temgoua et al. [21,26]. Nevertheless, Bagchi et al. recruited a higher proportion of males than females [25]. The differences in the proportion of males and females recruited into the various studies may be explained by the sampling techniques used. First degree relatives of CKD patients were voluntarily screened for CKD in Bagchi et al study, whereas this study used balloting method in selecting the FDRs [25].
The median age of the FDRs in this study was 36.0(24.0%) years. This was comparable to mean ages of 38.40±13.80 years, 39.80±14.70 years and 41.30±14.20 years reported by similar studies done by Wei et al, Bagchi et al and Tsai et al respectively [21,23,27]. CKD is more common among the elderly population because of reduced nephron number associated with ageing and increased prevalence of co-morbidities such as hypertension and diabetes mellitus prevalent among the aged [28]. The common practice in Nigeria is that the elderly CKD patients are accompanied to the hospital by their younger first degree relatives and this may explain the higher number of younger age group obtained in this study.
There was a higher level of education among the FDRs. Ninety six percent of FDRs had formal education, while only 4% had no formal education. About 72.7% of FDRs were employed, 26.7% were students and 0.7% was unemployed. This was comparable to findings of Ogiator et al who found formal education and being employed in 76% and 72% of FDRs respectively [11]. A higher proportion of FDRs (68.7%), earn above N20,000 (Naira).
Prevalence of CKD among FDRs of CKD patients
The prevalence of CKD among FDRs of CKD patients in this study was 26.7% and this was significantly higher than 9.3% observed in the control arm of the study. Furthermore, FDRs were about 4 times more likely to have CKD than the controls [OR (95% CI)=3.5(1.8-6.8)]. The higher prevalence of CKD among FDRs in this study was in agreement with previous Studies that showed that family relatives of patients with CKD were at increased risk of CKD [15].
The prevalence of CKD in this study was similar to 29.7% reported by Wei et al in Southern China [21]. Both studies adopted similar definition for CKD and estimation of eGFR and this could account for the similarity in prevalence observed.
On the other hand, the prevalence of CKD in this study was higher than the 11% reported by Ogiator et al in Northern Nigeria, 15.8% reported by Temgoua et al. in Cameroun and 19.3% reported by Elemam et al in Sudan [11,26,29]. Also the prevalence of CKD in this study was lower than 37.4% reported by Raji et al in Southwest Nigeria [19]. These differences in the prevalence could be attributed to the differences in the methodology, including the sample size and the characteristics of the populations studied. Ogiator et al. screened 100 relatives of patients with advanced CKD, Raji et al. studied 230 FDRs of CKD patients, Temgoua et al. studied 82 FDRs of haemodialysis patients while Elemam et al. screened 135 FDRs of haemodialysis patients [11,19,26,29]. The screening methods employed by the different studies could also account for the differences in the prevalence. These four studies screened for CKD only at baseline as opposed to this study that screened CKD at baseline and after 3 months in accordance with KDIGO 2012 criteria that defined CKD as reduced eGFR and/or markers of kidney function for more than three months [1]. These other studies did a cross sectional study and screened for CKD only at baseline.
Conclusion
The prevalence of CKD among FDRs of CKD patients in Nnewi, South Eastern Nigeria was high (26.7%).
Limitations
Genetic investigations to determine the genes implicated in development of CKD among FDRs were not done due to cost. The study was a hospital based one and so the findings might not be generalized. GFR was estimated using MDRD formula which might not be as accurate as using GFR measured by a standard method such as inulin clearance.
Declarations
Recommendations: Individuals with family history of kidney disease should be routinely screened for kidney disease at any contact with a medical practitioner. Health education of CKD patients regarding the high risk of developing CKD by their FDRs and the merits of screening them for CKD should be done by doctors at any contact with CKD patients. Genetic investigations to detect the genes associated with CKD in our local population should be explored.
Contributions of the study to knowledge: This study has added to the current knowledge on the prevalence of CKD among FDRs of CKD patients in Nigeria.
This study also underscored the need for appropriate counseling and early referral of FDRs of CKD patients with detected kidney disease to Nephrologists for expert management.
References
- KDIGO 2012 Clinical practice guidelines for the evaluation and management of CKD. Kidney Int Suppl. 2013; 3(1): 5-14.
- Vivekanand P, Dm J, Garcia-garcia PG, Iseki PK, Li Z, et al. Chronic kidney disease: Global dimension and perspectives. Lancet. 2013; 382(9888): 260-72.
- George C, Mogueo A, Okpechi I, Echouffo-Tcheugui JB, Kengne AP. Chronic kidney disease in low-income to middle-income countries: The case for increased screening. BMJ Global Health. 2017; 2(2).
- Collins AJ, Foley RN, Herzog C, Chavers B. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013; 61(1): 6386.
- Kaze AD, Ilori T, Jaar BG, Echouffo-Tcheugui JB. Burden of chronic kidney disease on the African continent: A systematic review and meta-analysis. BMC Nephrology. 2018; 19: 125.
- Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, et al. Global, regional and national burden of chronic kidney disease, 1990 - 2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020; 395(10225): 709-733.
- Ulasi II, Ijoma CK, Onodugo OD, Arodiwe EB, Ifebunandu NA, et al. Towards prevention of chronic kidney disease in Nigeria: a community-based study in Southeast Nigeria. Kidney Int Suppl. 2013; 3(2): 195-201.
- Afolabi MO, Abioye-Kuteyi EA, Arogundade FA, Bello IS. Prevalence of chronic kidney disease in a Nigerian family practice population. South African FAM Pract. 2009; 51(2): 132-7.
- Ulasi II, Ijoma CK. The enormity of chronic kidney disease in Nigeria: The situation in a teaching hospital in south-east Nigeria. J Trop Med. 2010; 2010: 501957.
- Chen SC, Huang JC, Su HM, Chiu YW, Chang JM, et al. Prognostic cardiovascular markers in chronic kidney disease. Kidney Blood Press Res. 2018; 43(4): 1388-407.
- Ogiator M, Agaba E, Agbaji O, Shaahu V. Prevalence of Chronic Kidney Disease in Relatives of Patients with Advanced Renal Disease in a Nigerian Tertiary Hospital. J Adv Med Med Res. 2017; 22(3): 1-6.
- Satko SG, Freedman BI, Moossavi S. Genetic factors in end-stage renal disease. Kidney Int Suppl. 2005; 67(94): 2001-4.
- Eikmans M, Aben JA, Koop K, Baelde HJ, de Heer E, et al. Genetic factors in progressive renal disease: The good ones, the bad ones and the ugly ducklings. Nephrol Dial Transplant. 2006; 21(2): 257-60.
- Li G. Genetic Factors for End-Stage Renal Disease. J Integr Nephrol Androl. 2015; 2(2): 46.
- Kazancioǧlu R. Risk factors for chronic kidney disease: An update. Kidney Int Suppl. 2013; 3(4): 368-71.
- Dummer PD, Limou S, Rosenberg AZ, Heymann J, Nelson G, et al. APOL1 Kidney Disease Risk Variants: An Evolving Landscape. Semin Nephrol. 2015; 35(3): 222-36.
- Friedman DJ, Pollak MR. APOL1 and Kidney Disease: From Genetics to Biology. Annu Rev Physiol. 2020; 82(1): 323-42.
- Chen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA - J Am Med Assoc. 2019; 322(13): 1294-304.
- Raji Y, Mabayoje M, Bello B, Amira C. Albuminuria and reduced estimated glomerular filtration rate among first-degree relatives of patients with chronic kidneydisease in Lagos, Southwest Nigeria. Indian Journal of Nephrology. 2018; 28: 21-7.
- Levey AS, Schoolwerth AC, Burrows NR, Williams DE, Stith KR, et al. Comprehensive Public Health Strategies for Preventing the Development, Progression, and Complications of CKD: Report of an Expert Panel Convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009; 53(3): 522-35.
- Wei X, Li Z, Chen W, Mao H, Li Z, et al. Prevalence and risk factors of chronic kidney disease in first-degree relatives of chronic kidney disease patients in Southern China. Nephrology. 2012; 17(2): 123-30.
- Gouda Z, Mashaal G, Bello AK, Attar A El, Kemmry T El. Egypt Information, Prevention, and Treatment of Chronic Kidney Disease (EGIPT-CKD) Programme: Prevalence and Risk Factors for Microalbuminuria among the Relatives of Patients74 with CKD in Egypt. Saudi J Kidney Dis Transpl. 2011; 22(5): 1055-1063.
- Slot C. Plasma creatinine determination a new and specific jaffe reaction method. Scand J Clin Lab Invest. 1965; 17(4): 381-7.
- Levey AS, Stevens LA, Frcp C, Schmid CH, Zhang YL, et al. A New Equation to Estimate Glomerular Filteration Rate.Ann Intern Med. 2009; 150(9): 604-12.
- Bagchi S, Agarwal SK, Gupta S. Targeted screening of adult firstdegree relatives for chronic kidney disease and its risk factors. Nephron - Clin Pract. 2010; 116(2): 128-136.
- Temgoua MN, Ashuntantang G, Essi MJ, Tochie JN, Oumarou M, et al. Prevalence and Risk Factors for Chronic Kidney Disease in Family Relatives of a Cameroonian Population of Hemodialysis Patients: A CrossSectional Study. Hosp Pract Res. 2019; 4(1): 12-7.
- Tsai JC, Chen SC, Hwang SJ, Chang JM, Lin MY, et al. Prevalence and Risk Factors for CKD in Spouses and Relatives of Hemodialysis Patients. Am J Kidney Dis. 2010; 55(5): 856-66.
- Nalado A, Abdu A, Adamu B, Aliyu MH, Arogundade FA, et al. Prevlaence of chronic kidney disease markers in Kumbotso rural Northern Nigeria. African journal of medicine and medical sciences. 2016; 45: 61-5.
- Elemam AM. Obese First-degree relatives of hemodialysis patients are at Higher Risk for Developing Kidney Diseases: In a Cross-sectional Study. Sudan J Med Sci. 2019; 14(3): 143-54.