Review Article
Volume 3, Issue 1
Are Freshwater Sources Safe for the Health of Humans and Domestic Animals in Terms of Deadly Trematodiases?
Shanti Lal Choubisa1*; Pallavi Choubisa2
1Department of Advanced Science and Technology, National Institute of Medical Science and Research, NIMS University Rajasthan, Jaipur, Rajasthan 303121, India; Former Department of Zoology, Government Meera Girls College, Udaipur, Rajasthan 313001, India.
2Department of Gynaecology and Obstetrics, RNT Medical College and Pannadhay Zanana Hospital, Udaipur, Rajasthan 313002, India.
Corresponding Author :
Shanti Lal Choubisa
Email: choubisasl@yahoo.com
Received : Dec 08, 2023 Accepted : Jan 12, 2024 Published : Jan 19, 2024 Archived : www.meddiscoveries.org
Citation: Choubisa SL, Choubisa P. Are Freshwater Sources Safe for the Health of Humans and Domestic Animals in Terms of Deadly Trematodiases?. Med Discoveries. 2024; 3(1): 1102.
Copyright: © 2024 Choubisa SL. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
There are many types of lentic and lotic freshwater sources on earth, such as puddles, ponds, rivers, springs, dams, lakes, etc. and many of them are found near human settlements. But most of the people are not known and aware of these freshwater sources how safe and dangerous they can be. In fact, these water sources are potentially responsible for spreading various deadly trematodiases (trematode parasitic diseases) to both humans and domestic animals. The most common zoonotic or vector-borne dreaded trematodiases found in humans and domestic animals are fascioliasis, schistosomiasis, paragonimiasis, echinostomiasis, amphistomiasis, etc. These parasitic diseases are endemic in almost every country. But these diseases can occur in any geographical area only when different species of snails (Mollusca: Gastropoda) and crabs or crayfish (Crustaceans) are found in the freshwater bodies there. In fact, these aquatic invertebrates are the intermediate hosts of diverse pathogenic digenetic trematode parasites including Fasciola hepatica, Schistosoma mansoni, Paragonimus westermani, Ceylonocotyle scoliocoelium, etc. and complete their life cycle. These are also known as vectors or carriers and are responsible for the transmission or spread of various trematodiases in different geographical areas. Therefore, these diseases are also called vector- borne diseases. Apart from causing varying levels of morbidity and mortality in humans and animals, these diseases also cause tremendous economic losses to livestock owners. However, it is not necessary that all types of freshwater sources are beneficial, but from the point of view of trematodiases, the water sources in which different types of snail species are found can also be dangerous or unsafe for the health of humans and domestic animals. But most of the people are ignorant and unaware of how these parasitic diseases transmitted or spread to humans and domestic animals through various freshwater bodies located around them. The current review highlights how freshwater sources can be hazardous or dangerous to the health of humans and domestic animals in the context of diverse trematodiases and also focused on their prevention and control.
Keywords: Digenetic trematode parasite; Domestic animals; Humans; Freshwater sources; Intermediate hosts; Ruminants; Snails; Trematode larvae; Trematodiasis.
Introduction
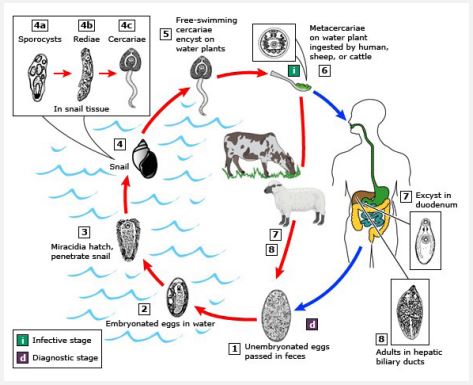
Many types of lentic (stationary) and lotic (floating) freshwater sources, such as puddles, ponds, rivers, springs, canals, dams, lakes, etc., are found on planet Earth and many of them are located in the vicinity of human and domestic animal populations. These small or large surface freshwater sources can also be shallow or deep and seasonal or perennial. The water of these sources is usually drunk by humans and domestic animals. In rural areas, most people and domesticated animals like cattle (Bos taurus), water buffaloes (Bubalus bubalis), sheep (Ovis aries), goats (Capra hircus), camels (Camelus dromedarius), etc. also bath and swim in the freshwater bodies. Herbivorous domestic animals also eat the various vegetation or plants growing in these freshwater sources while humans also eat or consume aquatic vegetables, fruits, fish, crabs, crayfish, etc. as food. During this time, generally, humans and domestic animals become infected with the highly infectious or infective trematode larvae, cercariae and metacercariae, of pathogenic digenetic trematode parasites (flukes). Infection with these larvae usually results in various trematodiases. Generally, people are not aware of this type of infection nor are they aware of the infection of these parasites in their domesticated animals. The most common pathogenic digenetic trematode parasites, such as liver flukes (Fasciola hepatica, F. gigentica, etc.), rumen/intestinal flukes (Orthocoelium scoliocoelium, Gastrothylax cruminifer, Megalodiscus temperatus, Cotylophorum cotylophorun, Ceylonocotyle scoliocoelium, Paramphistomum cervi, P. microbothrium, etc.), lung fluke (Paragonimus westermani), blood fluke (Schistosoma mansoni), urinary fluke (S. haematobium), etc. (Figure1) are found in humans and domestic animals [1,6]. Generally, digenetic trematode parasites are found to be strongly host-specific, but some may have more than one definitive host or a wide range of hosts [1,7,8]. They mostly infect the important vital organs like liver, alimentary canal, heart, eyes, lungs, etc. of both human and animal hosts. They cause a lot of harm or histological and physio-chemical changes or damages to them. Humans and animals also die due to their excessive or heavy infection. The most common trematodiases that are found in humans and animals are fascioliasis, amphistomiasis, paramphistomiasis, schistosomiasis, paragonimiasis, echinostomiasis, etc. These trematodiases can also be regionally-specific or widespread [1,6]. But some of them are commonly found in both humans and domestic animals. However, the endemicity of any trematodiasis depends on the availability of their intermediate hosts, snails and crab or crayfish [1,6].
Snail species inhabit most freshwater sources
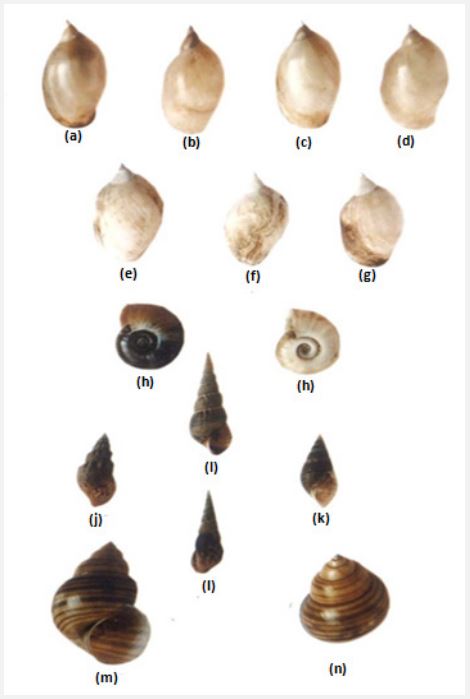
It is well established that in different lentic and lotic freshwater sources, such as puddles, ponds, rivers, springs, dams, lakes, etc. have their own type of ecosystem in which different species of snails are inhabited [7]. The most common and widely distributed snail species found in various freshwater bodies in different geographical regions generally belong to the families Lymnaeidae, Planorbidae, Thiaridae (Melanidae) and Viviparidae of the Class- Gastropoda of the Phylum- Mollusca. The most common snail species found in various freshwater sources in Asian countries are Lymnaea acuminate f. patula, L. acuminate f. chlamys, L. acuminate f. typica, L. acuminate f. rufescens, L. luteola f. australis, L. luteola f. typica, L. luteola f. impura, Gyraulus convexiusculus, Planorbis (Indoplanorbis) exustus, Faunus ater, Melania (Plotia) scabra, Thiara (Tarebia) lineata, Melanoide striatella tuberculata, Vivipara bengalensis race gigantica, V. bengalensis race mandiensis, etc. (Figure 2) [9-12]. Many of these snails are habitat-specific. Therefore, they have been considered as bio-indicators for various freshwater habitats and trematodiases [13,14]. These aquatic snails are also carriers or vectors of various trematodiases in humans and cattle and are responsible for the transmission of these trematode parasitic diseases. Therefore, these diseases are also called as snail-borne trematodiases. It is well known that almost all freshwater snail species are intermediate hosts of digenetic trematodes (flukes) parasites and complete their life cycle. In fact, in these snails, various larval stages of these pathogenic trematodes parasites develop called sporocyst, redia, and cercaria. Importantly, thousands of cercarial larvae can develop from a single sporocyst by following the mechanism of asexual reproduction. This is one of the parasitic adaptationsin trematode parasites by which they can survive and thereby save their species. Interestingly, these intra-molluscan larvae develop only in the vital organs of the snail hosts, the hepatopancreas (liver) and gonads (ovotestis).
Zoonotic trematodiases
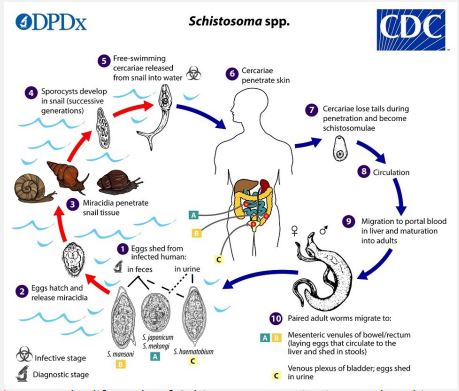
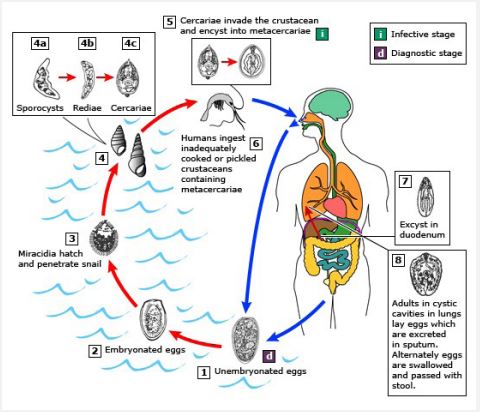
In humans and herbivorous domestic animals, various zoonotic or vector-borne trematodiases are caused by infection with diverse species of pathogenic digenetic trematode parasites. The life cycle of these parasites is complicated and is completed in two different species of hosts, primary or definitive (humans and mammalian animals) and secondary or intermediate (aquatic snails) hosts. However, in certain species of digenetic trematode parasite, such as P. westermani, the life cycle is completed in three different hosts instead of two, man/animals, snails, and crabs/crayfish. Their asexually developing larvae, sporocysts, redia, and crecariae are also found to be pathogenic to the snail hosts in which they develop and multiply. These trematode larvae cause varying degrees of pathogenesis in the vital organs of snails, hepatopancreas and gonads depending on degree of parasitemia [15,22]. Transmission of trematode parasites, generally, occurs through consumption of water and food contaminated with larval stages found in their life cycle, cercariae and metacercariae. Therefore, trematodiasis developed due to consumption of water and food contaminated with larval stages is known as water- and food-borne trematodiasis, respectively. In general, in a host organism, the eggs of trematode parasites are spread through feces or stool, but there are also species of trematode parasites, such as Schistosome sp. (blood fluke) and P. westermani (lung fluke) whose eggs are also spread through urine and sputum, respectively.
In fact, without aquatic snails, the life cycle of trematodes is not possible or completed, that is, if there are no aquatic snails in an area, there will be no trematode parasites or trematodiases. These snails are also potential for the transmission of various water-and food-born trematodiases from one geographical region to another. That is why to control trematodiasis in any geographical area or country; the first step is to control the snail population [7]. Interestingly, the intra-molluscan larval stages of trematode parasites develop in the snails also control the population of snails naturally by the mechanism of parasitic castration [17,21,23].
In general, almost all vertebrates including human beings and domestic animals are the primary or definitive hosts of almost all pathogenic digenetic trematode parasites. Thousands of fertilized eggs of these parasites are eliminated or released or excreted through the feces, saliva/sputum, urine, etc. of infected individuals (the primary hosts), depending on the species of trematode parasites. Ultimately, these eggs are carried by rainwater to various freshwater bodies where the eggs hatch into miracidium larvae, the first larval stage of trematode parasites. These free-swimming infective larvae eventually infect the intermediate host, the snail where they undergo asexual reproduction or multiplication and develop various parthogenic developmental stages, such as sporocyst, redia, and cercaria. In these intra-mollscun trematode larvae, cercarial larvae are free-swimming larvae and usually emerge from the snail host during the day hours. Cercariae of different species of trematode parasites have different anatomy and morphology. Based on their anatomy and morphology, these are also classified or named as monostome, xiphidio, furcocercous, amphistome, echinostome, gymnocephalous, transversotrematid cercariae, etc. [23,41]. The type of trematodiasis that is endemic in any area can be predicted or expected based on the types of cercariae as well as snail species [7].
Most of the cercarial larvae are highly active, good swimmers, phototrophic, and negative geotaxis [42,44]. They have also strong host- specificity [42,44]. These larvae have well developed organ systems, such as digestive system, excretory system, nervous system, etc. [45,52]. Whenever, while swimming, they come in contact with any objects or substratum (mostly leaves and twigs of aquatic plants, crabs, crayfish, fish, etc.), they stick to them where they undergo encystment or form a cyst around themselves. These encysted cercariae are called metacercariae larvae. Actually, these larvae are juvenile flukes or trematodes. These larvae are highly infective or infectious and can survive for a short time in unfavorable environments due to the presence of cysts around them. Whenever, these larvae enter the body of definitive hosts, humans and animals, through eating of aquatic foods contaminated with these larvae then they become excysted in the digestive system. Ultimately, the larvae reach their target organs in the body where they develop into adults and then start sexual reproduction and simultaneously also cause varying degrees of pathogenicity. Trematodiasis develops by eating raw or inadequately cooked aquatic foods, such as vegetables, fruits, crabs, crayfish, fish, liver of animals, etc. contaminated with metacercariae, then it is also called as food-borne trematodiasis (fascioliasis, amphistomiasis, paragonimiasis, etc). In some digenetic trematode parasites such as Schistosoma sp., their cercarial larvae are also infective and directly enter human and animal bodies through their skin and cause the fatal disease, schistosomiasis [1,4,5]. This type of trematodiasis developed by infection of cercarial larvae is called water-bone trematodiasis. A schematic representation of the life cycles of most of the common digenetic trematode parasites is shown in Figures 3-5. In these life cycles of trematode parasites, the intermediate host snail is common, but the life cycle of the trematode P. westermani (lung fluke) involves two intermediate hosts, the snail and the crab or crayfish (Figure 4).
The prevalence and endemicity of any kind of vector- borne or water- and food-borne trematodiasis in any geographical area depends on the infected snail population. Depending on the species of snail, it can also be predicted which type of trematodiasis will be endemic in area. Since each snail species completes the life cycle of a particular species of digenetic trematode parasite. In fact, those freshwater sources in which snail species are found are potential sources of transmission of various trematode parasitic diseases or trematodiases to humans and animals. Therefore, such freshwater sources are not safe for the health of humans and animals. But most of the people are unaware of this. Though, these trematodiases can easily be diagnosed by stool examination of definitive or primary hosts [53,56].
Prevention and control of trematodiases
Diverse water-borne or snail-borne digenetic trematode parasitic diseases or trematodiases, such as fascioliasis, amphistomiasis, schistosomiasis, paragonimiasis, echinostomiasis, etc. not only cause people and animals to fall sick and die but also cause huge economic losses in the country where these diseases are endemic. People who do animal husbandry business, when their animals suffer from these diseases, a lot of money is spent in their treatment and the death of the animals causes further financial loss. Therefore, prevention and control of trematodiases is more important and necessary. But most of the people or villagers are still unaware of these water or snailborne various dreaded trematode parasitic diseases. General awareness in both urban and rural populations is highly suggestive for the prevention and control of these parasitic diseases in humans as well as in their domesticated animals. Antiparasitic treatment, control of snail populations, improved sanitation and access to safe water, and communication about improved food safety and hygiene are important for the control of trematode parasitic infections. Interestingly, apart from water- and food-borne trematodiasis, people in many countries including India are also unaware of hydrofluorosis, a hyperendemic water-borne disease caused by chronic fluoride intoxication, which is prevalent not only in humans but also in their various species of domesticated animals (cattle, buffaloes, sheep, goats, camels, etc.) [57,73].
Conclusion
Most lentic and lotic natural freshwater sources, such as puddles, ponds, rivers, canal, springs, dams, lakes, etc., contain populations of different species of snails. In fact, these snails are intermediate hosts of diverse species of pathogenic digenetic trematode parasites and complete their life cycle. Various fatal trematodiasis, such as fascioliasis, amphistomiasis, schistosomiasis, paragonimiasis, echinostomiasis, etc. occur in vertebrates, including humans and animals, through infection with the infective larvae of digenetic trematode parasites developing in snails. Therefore, freshwater sources that contain various species of snails are hazardous to human and animal health as they are more likely to cause trematodiases in them. Any kind of trematodiasis not only causes morbidity and mortality in humans and animals, but also causes enormous economic losses in several ways. Therefore, prevention and control of these water-borne parasitic diseases is highly suggestive. General awareness, improved food safety, hygiene and sanitation, and health educatuion at school level are effective in prevention and control of these diseases. There is a great need to run a large scale campaign to control these diseases in the countries where these diseases are found in abundance or hyperendemic. This will also help directly or indirectly in maintaining the economic condition of these countries.
Declarations
Acknowledgment: The author thanks to Dr. Darshana Choubisa, Professor, Department Prosthodontics and Crown & Bridge, Geetanjali Dental and Research Institute, Udaipur, Rajasthan 313002, India for cooperation.
Funding: No funding was received for this work.
Competing interest: The authors have no conflict of interest.
References
- Cheng TC. General Parasitology, Academic Press, New York and London. 1973.
- Arora DR, Arora B. Medical Parasitology, CBS Publishers and Distributors Pvt. Ltd., New Delhi, India. 2010.
- Paniker CKJ. Paniker’s Textbook of Medical Parasitology, edited by Ghosh S, Jaypee Brothers Medical Publishers (P) Ltd, New Delhi, India. 2013.
- Khurana S, Mewara A. The Textbook of Medical Parasitology, Universities Press India Pvt. Ltd., Hyderabad, India, 2021.
- Bhatia BB, Pathak KML, Juyal PD. Textbook of Veterinary Parasitology, Kalyani Publishers, New delhi, India. 2021.
- Latha BR, Sundar STB, Dhivya B. Textbook of Veterinary Parasitology, Satish Serial Publications, Delhi, India. 2022.
- Choubisa SL. The Biology of Certain Larval Trematodes Infecting Freshwater Snails of Lakes of Udaipur. Ph.D. thesis, Mohanlal Sukhadia University, Udaipur, Rajasthan, India. 1984: 1-226.
- Erasmus DA. The Biology of Trematodes. Printed in Great Britain at the Universities Press, Belfast. 1972.
- Choubisa SL, Sharma PN. Seasonal variations of cercarial infection in snails of Fateh Sagar Lake of Udaipur. Indian J Parasitol. 1983; 7(1): 111-3.
- Choubisa SL, Sharma PN. Incidence of larval trematodes infection and their seasonal variation in the fresh water molluscs of southern Rajasthan. Rec Zool Surv India. 1986; 83(1&2): 69-80.
- Choubisa SL. Snail hosts of larval trematodes in Southern Rajasthan. Indian J Parasitol. 1991; 15(1): 49-51.
- Choubisa SL, Sheikh Z. A new variety of freshwater snail, Thiara scabra var. choubisai from Rajasthan, India. Cibtech J Zool. 2013; 3(3): 44-6.
- Choubisa SL. Mollusc as bio-indicators for the trophic stages of lakes and lotic environments. Bull Pure Appl Sci. 1992; 11A (1-2): 35-40.
- Choubisa SL, Sheikh Z. Freshwater snails (Mollusca: Gastropoda) as bio-indicators for ecologically diverse aquatic habitats. Cibtech J Zool. 2013; 3(3): 2-6.
- Choubisa SL. Histological and histochemical observations on the digestive gland of Melanoides tuberculatus (Gastropoda) infected with certain larval trematodes and focus on their mode of nutrition. Proc Indian Acad Sci (Anim Sci). 1988; 97(3): 251-62.
- Choubisa SL Histopathological observations on the digestive gland of Lymnaea auricularia infected with the larval trematodes. Proc Indian Acad Sci (Anim Sci). 1990; 99(5): 363-68.
- Lafferty KD. Effects of parasitic castration on growth, reproduction and population dynamics of the marine snail Cerithidea californica. Marine Ecol Prog Series. 1993; 96: 229-37.
- Choubisa SL. Focus on histopathogenesis of trematode larvae. J Parasit Dis. 1998; 22(1): 57-9.
- Choubisa SL, Sheikh Z, Jaroli VJ. Histopathological effects of larval trematodes on the digestive gland of freshwater snail species, Vivipara bengalensis and Lymnaea acuminate. J Parasit Dis. 2012; 36(2): 283-6.
- Choubisa SL, Sheikh Z. Parasitic castration in freshwater snail Melanoides tuberculatus (Mollusca: Gastropoda). Proc Natl Acad Sci, India Sect B: Biol Sci. 2013; 83(2): 193-77.
- Choubisa SL, Jaroli VJ. Freshwater larval digenetic trematode parasites in India: an epitomised review. Res J Chem Environm. 2020; 24(9): 146-56.
- Choubisa SL. A brief review of parasitic castration in aquatic snails and its contribution in control of diverse vector snail populations and trematodiases in man and animals. Austin J Infect Dis. 2022; 9(1): 1-6: 1066.
- Peter CT. Studies on the fauna in Madras. Part-II. A new species of echinostome cercariae. Indian J Vet Sci Anim Husb. 1955; 25: 219-24.
- Peter CT. Studies on the cercarial fauna in Madras IV. The amphistome and gymnocephalous group of cercariae. Indian J Vet Sci Anim Husb. 1956; 26: 27-30.
- Srivastava S. Studies on larval trematodes on a new echinostome cercaria, Cercaria tetraglandulata n. sp. from Indoplanorbis exustus. Proc Natl Acad Sci, India. 1963; 38: 230-34.
- Choubisa SL, Sharma SL. Cercaria tewarii n. sp. (Echinostomatid cercaria) from fresh water snail, Indoplanorbis exustus (Deshayes). Bio-Sci Res Bull. 1985; 1(1-2): 50-53.
- Choubisa SL. A gymnocephalous cercaria, Cercaria johrii n. sp. from fresh water snail, Melanoides tuberculatus (Muller) of Fateh Sagar Lake, Udaipur (Rajasthan). Indian J Parasit. 1985; 9(2): 245-47.
- Choubisa SL. Cercaria gurayai, n. sp. (furcocercaria) from the fresh water snail Faunus atter (Linnaeus). Rec Zool Surv India. 1990; 87(4): 267-71.
- Choubisa SL. On a rare cercaria, Cercaria udaipuriensis II n. sp. From the fresh water snail, Melanoides tuberculatus (Muller). Bio-Sci Res Bull. 1992; 8(1-2): 13-16.
- Pandey KC, Jain SP. A new amphistome cercaria from an Indian aquatic snail, Indoplanorbis exustus (Deshayes). Proc Zool Soc Calcutta. 1971; 24: 29-32.
- Mohandas A. Studies on the freshwater cercariae of Kerala VII. Echinostomatid cercariae. Proc Indian Acad Sci (Anim Sci). 1981; 90: 433-44.
- Saxena SK. A gymnocephalous cercaria, C. tandani sp. n. from Melanoides tuberculata (Muller). Helminthology. 1982; 19(3): 211-7.
- Sharma PN, Choubisa SL. Cercaria udaipuriensis n. sp. from fresh water snails, Vivipara bengalensis from Fateh Sagar Lake. Indian J Parasit. 1983; 7(2): 209-12.
- Choubisa SL. Focus on pathogenic trematode cercariae infecting fresh water snails (Mollusca: Gastropoda) of tribal region of southern Rajasthan (India). J Parasit Dis. 2008; 32(1): 47-55.
- Brinesh R, Janardanan KP. Three new species of xiphidiocercariae from the thiarid snail Thiara tuberculata in Palakkad, Kerala, India. J Parasit Dis. 2011; 35(1): 42-9.
- Choubisa SL, Sheikh Z. A rare trematode sporocyst from freshwater snail, Malanoides tuberculatus (Miller 1722). Cibtech J Zool. 2013; 3(3): 6-9.
- Sanil NK, Janardanan KP. Two new species of xiphidiocercariae from the apple snail Pila virens in Malabar, Kerala. J Parasit Dis. 2016; 40: 1614-9.
- Choubisa SL, Jaroli VJ, Sheikh Z. First record of a rare transversotrematid cercarial larva (Trematoda: Digenea) from Rajasthan, India: focus on seasonal occurrence and host-specificity of diverse cercariae. J Parasit Dis. 2017; 41(2): 496-502.
- Sanil NK, Janardanan KP. Furcocercous cercariae infecting freshwater snails in Malabar: two new species from Lymnea luteolaLamarck and Gyraulus convexiusculus (Hutton). J Parasit Dis. 2018; 42(2): 220- 25.
- Sanil NK, Janardanan KP. Four new species of virgulate xiphidiocercariae infecting the freshwater snail, Bithynia (Digoniostoma) pulchella (Benson, 1836) in Malabar, Kerala. J Parasit Dis. 2019; 43 (3): 368-78.
- Arusha, K, Prasadan PK. A parapleurolophocercous cercaria and a furcocercous cercaria from the freshwater gastropods of the Western Ghats. J Parasit Dis. 2019; 43(3): 479- 86.
- Choubisa SL. Comparative study on cercarial behaviours and their host specificity. Indian Journal of Parasitology. 1991; 15(2): 125-128.
- Choubisa SL. Seasonal variation of amphistome cercarial infection in snails of Dungarpur district (Rajasthan). J Parasit Dis. 1997; 21(2): 197-8.
- Choubisa SL. Focus on seasonal occurrence of larval trematode (cercarial) parasites and their host specificity. J Parasit Dis. 2002; 26(2): 72-4.
- Choubisa SL, Agrawal MP, Sharma PN. Histochemical distribution and functional significance of acetyl and butyryl cholinesterase in the amphistome Gastrothylax cruminifer. Proc Indian Acad Parasit. 1982; 3(1&2): 69-75.
- Choubisa SL, Sharma PN. Histochemical demonstration of cholinesterase in the nervous system of stregeoid metacercaria, Tetracotyle lymnaei. Indian J Parasit. 1983; 7(2): 217-9.
- Sharma PN, Choubisa SL. Histochemical demonstration of hydrolytic enzymes in two species of cercariae and in radia. Indian J Parasit. 1985; 9(2): 153-4.
- Choubisa SL. Histochemical demonstration of esterase in the certain fresh water larval trematodes with a note on neuroanatomy. Proc Indian Acad Sci (Anim Sci). 1986; 95(5): 623-8.
- Choubisa SL. Neuroanatomy of furcocercous, Cercaria milleri. Curr Sci. 1988; 57(7): 402-4.
- Choubisa SL. In-vitro culture of echinostome cercaria Cercaria tewarii (Choubisa and Sharma 1985) from the metacercaria to vitellogenous stage. Indian J Parasit. 1988; 12(1): 123-8.
- Choubisa SL. Distribution of non-specific esterase in certain larval digeneans with a note on morphology of nervous system. Indian J Experim Biol. 1989; 27(1): 32-57.
- Choubisa SL. Mode of nutrition in pathogenic trematode larvae (redia and cercaria) which infect hepatopancreas of fresh water snails (Mollusca: Gastropoda). J Parasit Dis. 2008; 32(1): 68-73.
- Choubisa SL, Choubisa L. Intestinal helminthic infections in tribal population of southern Rajasthan, India. J Parasit Dis. 2006; 30(2): 163-7.
- Choubisa SL. Parasitic infection in tribals and their domestic animals of southern Rajasthan. A technical report. Indian Council of Medical Research, New Delhi, India. 2011; 1-14.
- Choubisa SL, Jaroli VJ, Choubisa P, Mogra N. Intestinal parasitic infection in Bhil Tribe of Rajasthan, India. J Parasit Dis. 2012; 36(2): 143-8.
- Choubisa SL, Jaroli VJ. Gastrointestinal parasitic infections in diverse species of domestic ruminants inhabiting tribal rural areas of southern Rajasthan, India. J Parasit Dis. 2013; 37(2): 271-5.
- Adler P, Armstrong WD, Bell ME, et al. Fluorides and human health. World Health Organization Monograph Series No. 59. Geneva: World Health Organization. 1970.
- Choubisa SL. Chronic fluoride intoxication (fluorosis) in tribes and their domestic animals. Intl J Environm Stud. 1999; 56(5): 703-16.
- Choubisa SL. Some observations on endemic fluorosis in domestic animals of southern Rajasthan (India). Vet Res Commun. 1999; 23(7): 457-65.
- Choubisa SL. Endemic fluorosis in southern Rajasthan (India). Fluoride. 2001; 34(1): 61-70.
- Choubisa SL, Choubisa L, Choubisa DK. Endemic fluorosis in Rajasthan. Indian J Environm Health. 2001; 43(4): 177-189.
- Choubisa SL. Fluoridated ground water and its toxic effects on domesticated animals residing in rural tribal areas of Rajasthan (India). Intl J Environm Stud. 2007; 64(2): 151-9.
- Choubisa SL. Fluoride in drinking water and its toxicosis in tribals, Rajasthan, India. Proce Natl Acad Sci, India Sect B: Biol Sci. 2012; 82(2): 325-30.
- Choubisa SL. A brief and critical review of endemic hydrofluorosis in Rajasthan, India. Fluoride. 2018; 51(1): 13-33.
- Choubisa SL. A brief review of chronic fluoride toxicosis in the small ruminants, sheep and goats in India: focus on its adverse economic consequences. Fluoride. 2022; 55(4): 296-310.
- Choubisa SL. Endemic hydrofluorosis in cattle (Bos taurus) in India: an epitomised review. Intl J Vet Sci Technol. 2023; 8(1): 001-007.
- Choubisa SL. Chronic fluoride poisoning in domestic equines, horses (Equus caballus) and donkeys (Equus asinus). J Biomed Res. 2023; 4(1): 29-32.
- Choubisa SL, Choubisa D, Choubisa A. Fluoride contamination of groundwater and its threat to health of villagers and their domestic animals and agriculture crops in rural Rajasthan, India. Environm Geochem Health. 2023; 45:607-28.
- Choubisa SL. Is drinking groundwater in India safe for domestic animals with respect to fluoride? Arch Anim Husb Dairy Sci. 2023; 2(4): 1-7.
- Choubisa SL. Is drinking groundwater in India safe for human health in terms of fluoride? J Biomed Res. 2023; 4(1): 64-71.
- Choubisa SL. A brief and critical review of endemic fluorosis in domestic animals of scheduled area of Rajasthan, India: focus on its impact on tribal economy. Clinic Res Anim Sci. 2023; 3(1): 1-11.
- Choubisa SL. Is it safe for domesticated animals to drink fresh water in the context of fluoride poisoning? Clinic Res Anim Sci. 2023; 3(2): 1-5.
- Choubisa SL. Can people get fluorosis from drinking water from surface water sources? Fluoride test of water mandatory before its supply. SciBase Epidemiol Public Health. 2023; 1(2): 1006.