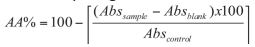Research Article
Volume 3, Issue 1
Antioxidant and Anti-Inflammatory Activity of Fractionated Artemia Urmiana Extract in Human Peripheral Blood Neutrophils System
Abdolkhalegh Deezagi*; Parisa Shahram
Department of Molecular Medicine and Biochemistry, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran.
Corresponding Author :
Abdolkhalegh Deezagi
Email: deezagi@nigeb.ac.ir
Received : Dec 12, 2023 Accepted : Jan 10, 2024 Published : Jan 17, 2024 Archived : www.meddiscoveries.org
Citation: Deezagi A, Shahram P. Antioxidant and Anti-Inflammatory Activity of Fractionated Artemia Urmiana Extract in Human Peripheral Blood Neutrophils System. Med Discoveries. 2024; 3(1): 1101.
Copyright: © 2024 Deezagi A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Marine organisms are great resources for discovering bioactive compounds with new antioxidant properties. The aim of this work was to purify and isolate the protein fractions of Artemia urmiana and to evaluate antioxidant activities of them on human peripheral blood neutrophils response. For these purposes, crude extracts protein was extracted from napulii of A. urmiana and sequentially fractionated by ion exchange. The antioxidant activities of the samples were determined by DPPH free radical scavenging, reducing power ability assays in comparison with ascorbic acid. In the second phase, the human peripheral blood neutrophils (HPBN) were separated and cultured. The cells were treated with increasing concentrations of crude and partially purified fractions for 24 hrs. After that, the pellet and supernatant of treated cells were separately collected and assayed for Nitro Blue Tetrazolium (NBT) reduction, the activity of Super Oxide Dismutase (SOD) and Nitric oxide content. The results showed that the IC50 values of ascorbic acid, crude extracts, partially purified protein fractions(F1-F8) were 150, 126, 141, 295, 299, 114, 164 and 132 µg/ml respectively in the DPPH radical scavenging activity. The reducing power activity at the highest concentration (200 µg/ml) based on the optical density of the sample listed, were 2.8, 1.4, 0.13, 0.14, 0.17, 0.14, 0.13, 0.66, 0.18 and 0.35 respectively. In NBT reduction and production of Nitric oxide, treated cells did not show significant difference between different concentrations of protein extract and untreated control cells. The activity of SOD was increased in presence of 50-100 µg/ml of crud extract and fractions 1, 2, 3, 4 and 5 in comparison to untreated control cells (p<0.05).
Keywords: Artemia urmiana extract; Antioxidant; Anti-inflammatory; Neutrophils; Super oxide dismutase.
Introduction
Antioxidants are compounds that protect the body against damage caused by oxidative stress that induce by free radicals. Reactive oxygen species (ROS), including free radicals, are formed by exogenous chemicals and endogenous metabolic processes in the human body. It is well known ROS such as •O2 (superoxide anion), H2O2 (hydrogen peroxide), and •OH (hydroxyl radical) are closely involved in various human diseases such as Alzheimer’s disease, aging, cancer, inflammation, rheumatoid arthritis and atherosclerosis [1,2]. There is a balance between the production of free radicals and the body’s antioxidant system, and if the production of free radicals increases or the antioxidant system weakens, nitrous or oxidative stress is created. Damage to macromolecules in the cell can include DNA strand breakage, peroxidation of cell membrane lipids, damage to transporter proteins in the membrane, and damage to intracellular enzymes [3,4].
Because oxidative stress is defined as an increase in the production of free radicals that lead to tissue damage. There are many potential sources of free radical production in the body, and one of these sources is activated neutrophils [5]. Neutrophils produce reactive oxygen species (ROS) and reactive nitrogen species (RNS), a key component of the neutrophil’s defense mechanism against invasion by foreign pathogens and parts of damaged host tissue [6].
For several years, many researchers have been searching for powerful but non-toxic antioxidants from natural sources, especially medicinal plants and marine organisms [7]. Furthermore, marine organisms are great resources for discovering bioactive compounds with new antioxidant properties. Also, antioxidants play an important role in nutritional by lengthening the shelf life of food and reducing nutritional losses and formation of harmful substances [8]. Thus, attention is now increasingly paid to the development and utilization of more effective and non-toxic antioxidants of natural origin. A great number of natural medicinal plants and marines have been tested for their antioxidant activities and results have shown that the raw extracts or isolated pure compounds from them were more effective antioxidants in vitro than BHT or vitamin E and C [9,10].
Artemia found in a wide variety of hyper saline habitats ranging from desert to, tropic to, mountains [11]. Artemia encysted and diapause exhibits a level of stress tolerance such as hyper salinity, very low oxygen tensions and extreme of temperature [12]. Species of this genus are found in a variety of very harsh environments in all continents, except Antractica. One of this species lives in Urmia lake in Iran. Lake Urmia (or Orumiyeh), is one of the largest permanent hypersaline lakes in the world and resembles the Great Salt Lake in the western USA in many respects of morphology, chemistry and sediments [13]. Historical overview of Artemia population from Iran was discussed in detail by Abatzopoulos et al. [14].
In nature their encysted embryos (Cysts), encounters sever hyper salinity and air desiccation; high dose of ultraviolet radiation; varying degree of hypoxia, inducing anoxia and extremes of temperatures [15]. Information’s related to varied uses of several species of the genus Artemia were existed. Nauplii (newly Artemia hatched) nutritional properties make it most widely used in aquaculture as food item [16]. This organism used as remedies in folk medicine in Iran. Also, it has been reported that Artemia proteins confer the anti-aging activity in human fibroblast cells and induce differentiation and apoptosis in human leukemic HL-60 cells [17,18]. Artemia proteins affected on the cells under stress, but the molecular mechanism of these proteins has not been studied clearly in human cells in normal condition. The sea water and mud of Urmia lake have been used by Iranians for treatment of some diseases from many ancients ago. The source and origin of effective materials of them were not clear and did not study to now. As mention about one of the most living organism in that lake is Artemia Urmiana.
For this purpose, crude extracts of protein were extracted after separation of napulii from cysts of Artemia urmiana. Then the crude extracts were sequentially fractionated by ion exchange chromatography and the total protein of each fraction was determined by the Bradford method. Then the antioxidant activities of the samples were determined by two different test systems, namely, DPPH free radical scavenging and its reducing power ability assays and the results were compared with antioxidant ascorbic acid. In the second phase, after the separation of human peripheral blood neutrophils, the cells were treated with increasing concentrations of protein extracts (containing crude extract and all of protein Fractions isolated by column chromatography) of Artemia for up to 24 hours, then the cells and supernatant of protein extracts treated cells were collected separately. The effect of protein extract of Artemia on ability of Nitro Blue Tetrazolium (NBT) reduction, the activity of the enzyme Super Oxide Dismutase (SOD) and content of Nitric oxide were evaluated.
Material and methods
Cyst hatching: Artemia urmiana cysts were provided from Urmia lake (West-North of Iran). 10 gr of cysts were hydrated in tap and sea water at room temperature for one hour. Then the cysts were encapsulated by Sodium Hypochlorite (NaOCl 5% W/V) until the cysts color was changed from brown to orange (Approximately 2-3 minutes). Then the cysts were washed by 500 ml of cold distilled water. For hatching, the encapsulated cysts were feeded in 2 liters of artificial sea water (0.4 M NaCL, 0.009 M KCL, 0.05 M MgCL2, 0.009M CaCL2 and 0.028 M Na2SO2, pH=8) that reported by Liu and Mclennan [19]. The cysts were separately incubated at room temperature and 37 C° for 24 hrs under light chamber in a shaking incubator. Freshly hatched naupliies are phototropism, the napauliies were collected by attraction to light. The collected napuliies were washed with 400 ml of cold distilled water. Embryos were suspended in 50 ml of stock solution A (8) pH=7.4 and centrifuged at 3000 rpm for 20 minutes. The protein content of napuliies were extracted by different methods such as: sonication, 3 times freezing and thawing and homogenization.
Protein extraction and purification: 1 gr of freshly isolated naupuliies was dissolved in 5 ml of extraction buffer Tris- HCl (50 mM, pH 6.8). Liquid homogenization, high frequency sound waves sonication and 3 times freeze/thaw cycles in liquid nitrogen were used for protein extraction. The homogenate was filtered through two layers of Mira cloth into a 50 mL Falcon tube at room temperature. The filtered homogenate was keep at -20 C for protein purification. In all of experiments total protein concentration was measured by modified Bradford methods.
The proteins content of crude extracts was precipitated with salt extraction by using Ammonium Sulfate 40% (W/V) at 4°C by shaking for 2hr. The precipitate was centrifuged at 5000 rpm for 20 minutes for 20 min at 4°C. Then the pellet was dissolved in dialysis buffer (Tris-HCl 50 mM, NaCl 20 mM, pH 6.5) and dialyzed against the same buffer for 24 hr at 4°C by 2 times change of the buffer.
A 25×3 cm DE-52 column (Whatman) was used for chromatography. First, for equilibration, the column was extensively washed by binding Tris buffer (Tris-HCl 50 mM, NaCl 20 mM pH 6.5). Subsequently, protein was eluted applying elution buffer (100 ml of Tris-HCl 50 mM, NaCl 20 mM pH 6.5). Then salt gradient was applied by adding 400 ml of 0.1 to 1.0 M of NaCl in Tris-HCl 50 mM buffer pH 6.5. The samples were collected as 2.0 ml fraction by flow rate about 0.5 ml/min. The samples were monitored at 280 nm continuously. The samples of each fraction were collected and dialyzed. The final total protein concentration was measured by modified Bradford methods. Finally, the fractions were concentrated and sterilized by 0.2 µm filter papers (whatman) to use for further analysis in cell culture system.
SDS-PAGE electrophoresis was done for analyses the protein profile of purified fractions. For analysis, crude extract and different fractions of chromatography were electrophoresed on 13% resolving SDS-PAGE gel. The electrophoresis was done by LKB-Pharmacia electrophoresis system (LKB Pharmacia, Uppsala, Sweden). After running; the gels were stained by coomassie blue.
DPPH anti-oxidant activity assay: The percentage of antioxidant activity (AA%) of each substance was assessed by DPPH free radical assay. The measurement of the DPPH radical scavenging activity was performed according to methodology described by Mensor et al [20]. The samples were reacted with the stable DPPH radical in an ethanol solution. The reaction mixture consisted of adding 0.5 mL of sample, 3 mL of absolute ethanol and 0.3 mL of DPPH radical solution 0.5 mM in ethanol. When DPPH reacts with an antioxidant compound, which can donate hydrogen, it is reduced. The changes in color (from deep violet to light yellow) were read [Absorbance (Abs)] at 517 nm after 100 min of reaction using a UV-VIS spectrophotometer (Shimadzu Inc. Kawasaki, Japan), The mixture of ethanol (3.3 mL) and sample (0.5 mL) serve as blank. The control solution was prepared by mixing ethanol (3.5 mL) and DPPH radical solution (0.3 mL). The scavenging activity percentage (AA%) was determined by using the formula:
Measurement of reducing power: The reducing capacity of compounds generally depends on reductants, which, as a potential antioxidant by breaking down a free radical chain by hydrogen atoms, delay the lipid peroxidation process. In this method substances that have regenerative potential, they convert potassium ferric cyanide (Fe3+) to potassium Ferro cyanide (Fe2+) and then, in reaction with ferric chloride, form the ferric ferrous complex, which causes the formation of this complex. The color change of the solution from yellow is associated with varying degrees of green and blue, and the intensity of the color depends on the potential for antioxidant activity. This dye has the highest absorption at a wavelength of 700 nm. The reducing power of compounds is measured by the amount of light they absorb at the same wavelength. The reducing power of crude extract and purified fractions was determined using the method described previously by Zou [21]. A serial dilution of the extract was performed (200, 100, 50, 25 and 12.5 μg/mL) in 0.2 M phosphate buffer pH, 6.6 containing 1% ferrocyanate. The mixture was incubated at 50°C for 20 minutes. 10% trichloroacetic acid (TCA, 2.5 mL) was added to a portion of this mixture (5 mL) and centrifuged at 3,000 g for 10 minutes. The supernatant was separated and mixed with distilled water (2.5 mL) containing 1% ferric chloride (0.5 mL). The absorbance of this mixture was measured at 700 nm. The intensity in absorbance could be the measurement of antioxidant activity of the extract.
Clotting limulus amoebocyte lysate (LAL) assay: Determination of endotoxin contamination in all samples were conducted by LAL assay kit (sigma, St. Louis, MO, U.S.A.) according to the manufacture’s instruction. The samples were initially pretreated by boiling. The samples with lower than <0.1 ng/ml of endotoxin were used for human neutrophils treatment.
Isolation of human blood neutrophils: Human peripheral blood was obtained from healthy donors with informed consent. Blood was drawn into citrate-dextrose buffer (1:9; 100 mM sodium Citrate, 130 mM glucose, pH 6.5). hpPMN and hpMN cells were prepared by using a polymorpho-nuclear isolation kit according to the manufacturer’s instruction (Polymorphoprep-Nycodenz). In brief 5 ml of freshly prepared anti-coagulated whole blood was layered over 3.5 ml of polymorphoprep solution in a 12 ml centrifugal tube. The layered sample over polymorphoprep was centrifuged at 500×g for 30 minutes in swing out rotor at 22˚c. After centrifugation the 2 leukocyte bands were separated carefully. The top band at the sample/medium interface would consist of mononuclear cells and the lower band of polymorphonuclear cells. The cells were diluted by addition of one volume of culture medium at 0.5 normal concentration of medium in order to restore normal osmolality. The cells were spun down (at 22˚c, 400×g, 10 min). They were resuspend in the medium, spun down again and then were resuspended in culture medium. Monolayer cells slides were prepared by Cytospin (Shandow) and stained by Geimsa staining method. The purity of neutrophils was >95%. The cells were counted and assessed for viability by trypan blue.
Treatment of cells: The human blood neutrophils(2×105 cells/ mL in RPMI 1640) were separately treated by increasing concentration (0,1,5,10,50 and 100 µg/ml) of sterile Artemia urmiana crude extract and/or partially purified fractions contents in 5 mL of RPMI-1640 medium and 10% of FCS in 6-wells plates (NUNC, Denmark). The cells were incubated at 37°C for 24 hr. Then the cells and supernatant of the treated neutrophils were collected separately. The effect of protein extract of Artemia on ability of Nitro Blue Tetrazolium (NBT) reduction was assessed in neutrophils in pellet. The activity of the enzyme Super Oxide Dismutase (SOD) and content of Nitric oxide were evaluated in supernatants as describe below.
NBT reduction assay and spectrophotometric determination of solubilized formazan deposits: NBT test is a simple method to examine the reduction potential of neutrophils. As neutrophils sterilize consumption of the energy increases, the power of hexosephosphate shunt glucose metabolism strengthens. Glucose-6-phosphate decomposed from glucose is oxidized and produce pentose. The hydrogen released from the reaction is accepted by NBT stain in the phagosome. Then the NBT stains reduce to granule or spot black formazan, deposit in neutrophils plasma. Colorimetric NBT reduction assay was done as the method which described by Rice-Evans C.A. [22]. Briefly, 200 µl of the treated cell suspensions were transferred to corresponding wells in a 96 microwells, centrifuged at 3000 RPM for 15 min and washed once with 250 µl PBS. The cells were resuspended in 100 µl of RPMI+20% FCS then 100 µl of freshly prepared NBT solution (1 mg/ml in PBS) was added to each well and incubated 45 min at 37˚C. The microwells were placed on ice for 5 min. The reaction mixtures were then spun for 15 min at 3000 rpm, and supernatant discarded. The cells were washed in PBS and resulting cell pellets were resuspended, fixed and washed in 70% (V/V) methanol for 5 min. The methanol in addition to fixing the cells removes any unreduced NBT which may have adsorbed onto the microwells. The cell pellets were resuspended in 100 µl 2 M potassium hydroxide, with the aid of vigorous vortexing, the cells rapidly lyse, realizing the formazan into the potassium hydroxide. 125 µl DMSO was added to each sample, which results in the development of an intense turquoise color in positive samples. After 30 min the tray was placed in the plate reader and the absorbance of each sample was read at 620 nm.
Superoxide dismutase (SOD) activity: Superoxide Dismutase (SOD) catalyzes the dismutation of the superoxide radical (O2 -) into hydrogen peroxide (H2O2) and elemental oxygen (O2 ) and as such provides an important defense against the toxicity of the superoxide radical. In fact, over expression of SOD protects human neutrophils. The assay is based on the ability of superoxide dismutase to inhibit the reduction of nitro-blue tetrazolium by superoxide. Conversion of NBT to NBT-diformazan, which absorbs light at 560 nm. SOD reduces the superoxide ion concentration and thereby lowers the rate of NBT-diformazan formation. The extent of reduction in the appearance of NBTdiformazan is a measure of SOD activity present in an experimental sample. The assay is free of interference by other catalytic activities and is ideal for assaying SOD in mammalian cell ysates. This method is based on the method which described by Cheng et al. [23]. Briefly, 50 µl of cell suspension (with density 20,000 cell/well) was added in 96 flat-bottomed wells. These cells were treated with increasing concentrations of crude extract and Artemia protein components (0-100 µg/ml). After 24 hours of incubation, the cells were centrifuged and the cell pellet was washed with potassium phosphate buffer. The cell precipitate is suspended in 250 µl of potassium phosphate buffer and then these cells are freeze-thawed three times and then 20 µl of solution and 10 µl of NBT solution were added to each well. The plate was placed on a special white light lamp box for 5-8 minutes. Then 5 µl of Riboflavin was added and absorbance was measured at 540 nm (Zero time). The plate was re-incubated on the lamp box for 12 minutes and the absorbance of the samples was measured at 540 nm after 12 minutes by ELIZA plate reader. Zero-time absorbance is deducted from 12-minute absorbance for kinetic assay of SOD.
Measurement of the nitric oxide generation: Human peripheral neutrophils were treated by Artemia extracted and/ or partially purified fractions as described above. Cell-free supernatants were recovered after incubation. Produced NO was quantitatively measured as NO3 - (Nitrate: a stable metabolite of NO) and NO2- (Nitrite) concentrations by using enzymatic and colorimetric NO assays according the methods described by Schmidt [24] and Granger et al [25]. In this method, we used reduction of nitrate by commercially Aspergillus Niger nitrate reductase and measurement of the product (Nitrite) by Griess reagent in micro titer plate. In brief, 50 µl of cells condition medium or standards were added to each well. Just prior to each assay freshly made solutions of NADPH (0.02 M) (Sigma, St. Louis, MO, USA) 10µl, mixed solution of Glucose-6- Phosphate 50mM (Sigma, St. Louis, MO, USA) and Glucose-6- Phosphate Dehydrogenase (100 U/ml) 23 µl (Sigma, St. Louis, MO, USA), Nitrate Reductase 0.1 U/ml, 10 µl (Sigma, St. Louis, MO, USA) and 7 µl of Tris buffer 1.0 M pH 7.5 were prepared separate and added to related wells. Reactant were mixed finely and incubated at room temperature for 30 min. After that 100µl of freshly prepared Greiss reagent, (1% Sulfanilamide (Sigma, St. Louis, MO, USA) and 0.1% Naphtylenediamine (Sigma, St. Louis, MO, USA) in 2.8% of ortho-phosphoric acid) was added to each well and incubated at room temperature for 10 min Finally the absorbance was measured at 550 nm by ELISA reader (Labsystems Multiskan, Roden, Netherlands).
Statistical analysis: Each experiment was minimally performed three times for all data, each carried out in duplicated sequences. Data were analyzed using a One-Way Analysis of variance (ANOVA) Values were given as the mean ± Standard Deviation (SD) and analytical variables were compared by using the students’ t-Test. By convention, a α-level of p<0.05 was considered to be statistically significant.
Results
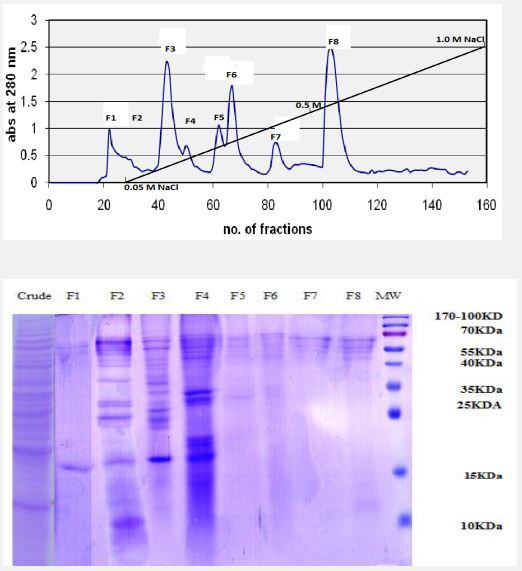
Protein extraction and purification and SDS-PAGE: Marine organisms are great resources for discovering bioactive compounds with new antioxidant properties. The aim of present work was to purify and isolate the protein fractions of Artemia urmiana and to evaluate antioxidant activities of its samples on human peripheral blood neutrophils. For these purposes, crude extracts of protein were extracted from napulii of A. urmiana by Homogenization and grinding. Then the total protein of extract was determined by the Bradford method. The crude extracts were sequentially fractionated by ion exchange chromatography and the total protein of each fraction was determined by the Bradford method. DE52 resin has been used to purifying and fractionation of extract. Figure 1A shows the chromatogram according optical density at 280 nm of the collected samples. As shown after load of sample, initially the column was washed with Tris-base buffer) until tube 55 and collected as fraction 1(F1). After that the NaCL gradient was applied from 0.1 to 1.0 M in Tris buffer sequentially which resulted 7 fractions (F2-F8). The total protein of each fraction was determined by the Bradford method and electrophoresis was done by using SDS-PAGE method. Figure 1B shows SDS-PAGE electrophoresis of Artemia crude protein extract and its protein fractions. In order to determine the quality and purity of fractions, equal amount (about 100 µg protein content) from each fraction in Tris-base buffer was mixed by sample buffer and boiled and was electrophoresis on Acrylamide-13% after cooling. The crude extract contains all the contact proteins of A. Urmiana. Protein groups with molecular weights of 15 and 55 kDa at peak F1, protein groups with molecular weights of 10 and between 15 and 70 kDa at peak F2 were removed from the chromatographic column, and at peak F3 protein groups with Molecular weights between 15 to 70 kDa, at peak F4 protein groups with molecular weight between 15 to 35 and 40 to 70 kDa, at peak F5 protein groups with molecular weight between 25 to 35 and 55 to 70 KDa, at peak F6 protein groups between 25 and 35 and 55 to 70 kDa, at peak F7 protein groups with a molecular weight between 55 and 70 kDa, at peak F8 protein groups with a molecular weight between 10 Up to 15 and 55 to 70 kDa were isolated.
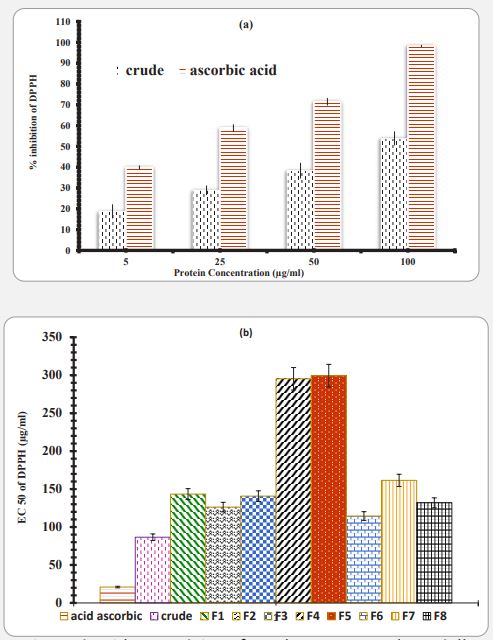
Anti-oxidant and reducing capacity: The crude extract and collected fraction samples were dialyzed against Tris buffer and equilibrated for removing of excess NaCl content. As described in material and methods, in DPPH assay, the results are expressed in terms of the percentage reduction in the rate of absorption of DPPH solutions in the presence of sample tests compared to DPPH solution alonely. Figure 2a compares the free radical scavenging activity of DPPH by Artemia umiana crude extract with ascorbic acid and shows that ascorbic acid has higher antiradical activity. Also, statistical analysis of these results under One-way ANOVA showed that increasing the concentration has a significant effect on free radical scavenging activity in a dose dependent manner. All of fractions were assayed for DPPH assay for dose response as crude extract (Data were not shown). In general, one-way analysis of variance of results from all fraction showed that the increasing of concentration has a significant effect on free radical inhibition activity. The linear equation for calculating the amount of IC50 for crude extract, fractions and ascorbic acid were done used and IC50 was calculated for each sample (Figure 2b). As shown in Figure 2b, the IC50 for the standard ascorbic acid and crude artemia extract in free radical scavenging is 62.9 and 21.6 µg/ml respectively. IC50 number for protein fractions F1, F2, F3, F4, F5, F6, F7 and F8, was 150, 126, 141, 295, 299, 114, 164 and 132 µg/ml respectively. In comparing, F6 showed the highest (114) and F5 the lowest (299) antiradical properties, respectively.
The reducing capacity of compounds generally depends on reductants, which, as a potential antioxidant by breaking down a free radical chain by hydrogen atoms, delay the lipid peroxidation process. The color change of the solution from yellow is associated with varying degrees of green and blue, and the intensity of the color depends on the potential for antioxidant activity. This dye has the highest absorption at a wavelength of 700 nm. The reducing power of compounds is measured by the amount of light they absorb at the same wavelength.
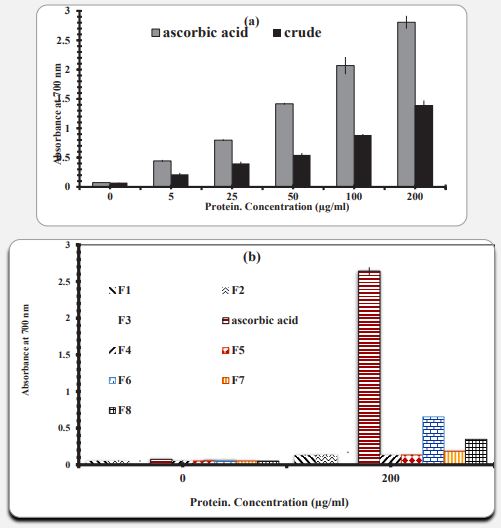
Figure 3a shows the regenerative capacity of Artemia umiana crude extract and ascorbic acid. The standard antioxidant ascorbic acid showed a higher regenerative capacity than the crude extract. Statistical analysis of these results under ANOVA shows that increasing the concentration of ascorbic acid and crude extract has a significant effect on regenerative power and with increasing concentration, increasing regenerative power is observed. Based on experiments performed to evaluate the reducing power of all fractions, concentrations less than 100 µg/ml showed low reduction power compared to the control. Therefore, we were only evaluating the reduction capacity of all protein fractions at a selective concentration of 200 µg/ml with three replications (Figure 3b). The result shows the reduction capacity of Artemia crude extract and its fractions, respectively. The reducing power of crude extract and ascorbic acid at the highest concentration (200 µg/ml) is 80.2 and 39.1, respectively. Reducing power of protein fractions F1, F2, F3, F4, F5, F6, F7 and F8 in the same concentration of 0/130, 0/136, 0/169, 0/139, 0/133, 0/654, respectively, 183 and 0/342. Therefore, the results show that Artemia crude extract has more regenerative power than its own fractions. In comparing the reducing capacity of all fractions at a concentration of 200 g/ml, from lowest to highest are F4, F1, F5, F2, F3, F7, F8 and F6, respectively. Therefore, F6 and F4 showed the highest and lowest regenerative properties, respectively.
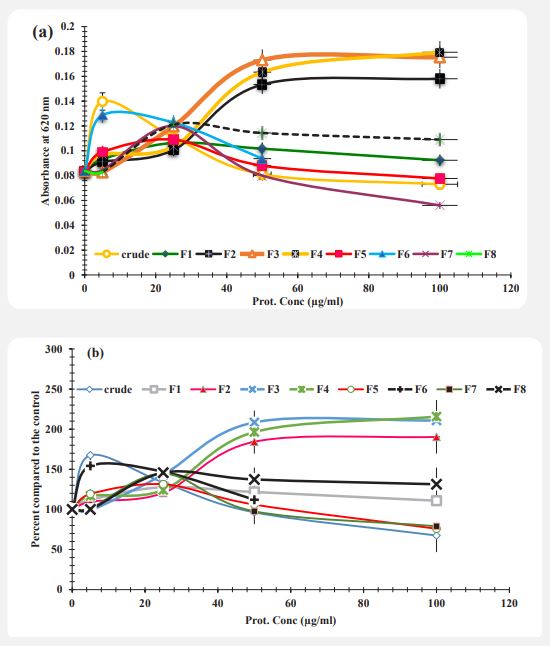
NBT reduction potential of neutrophils: After treatment of neutrophils by Artemia Urmian extract fractions, the potential of NBT reduction was assayed as described in methods. The soluble NBT dye which was reduced to un-soluble diformazan (NBD) solubilized by KOH and DMSO. The absorbance was measured at 620 nm and diagram was drawn. The effects of crude extract and protein fractions is shown in Figure 4a. The result indicates that the ability of cells in reduction of NBT in presence of crude extract and fractions (F1, F5, F6, F7 and F8) was decreased with increasing concentration. The reduction of NBT in neutrophil cells which treated with protein fractions F2, F3, F4 was increased with increasing concentration (p<0.05).
In order to study more accurately the effect of different concentrations as well as different components isolated from the column on the ability to reduce NBT in neutrophil cells, absorption diagrams based on concentration were calculated as a control percentage and the corresponding diagram was drawn. The amount of light absorption of the control sample cells (sample with zero concentration of Artemia extract) was calculated as 100% and the control percentage (by dividing the amount of light absorption of each sample by the amount of light absorption of the control sample). In (Figure 4b) each of the concentrations tested, different effects of each component were observed, at a concentration of 5 μg / ml F3 fraction, at a concentration of 25 μg/ml F2 fraction, at a concentration of 50 μg/ml and 100 μg/ml crude extract is more effective than other components in reducing the ability to reduce NBT.
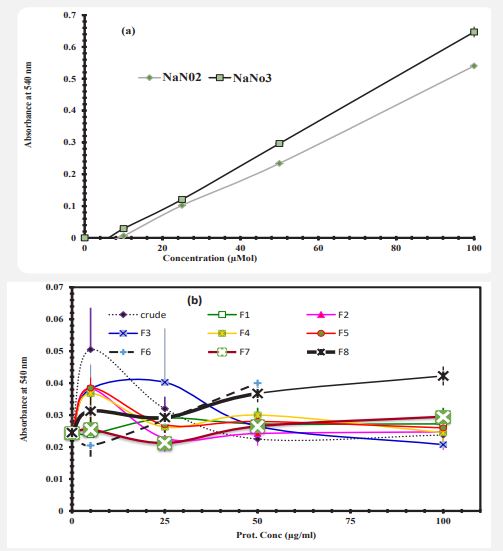
Nitric oxide production by neutrophils: In order to evaluate the amount of NO released in cell culture medium, standard curves are needed for the final nitric oxide products, which include nitrite and nitrate. To draw a standard curve (Figure 5a), the amount of Sodium Nitrite and Sodium Nitrate were measured as described in methods. Neutrophils were incubated with increasing concentrations of Artemia crude extract and fractions for 24 hours and then the concentration of NO was measured. The results are shown in Figure 5b. The results indicate that in stress-free conditions the cells which treated with different concentrations of crude extract and protein fractions F1, F2, F3, F4, F5, F6, F7 and F8 did not show significant differences with control cells. The cells which treated with higher concentrations, the production of NO was very low.
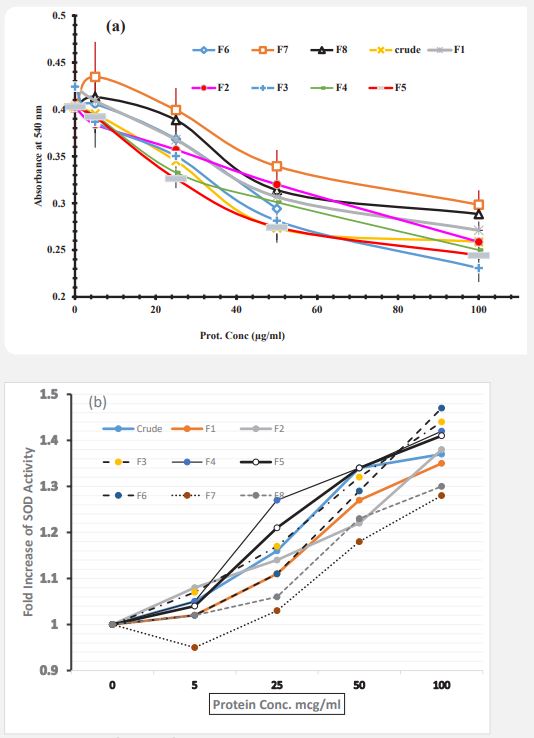
Super-oxide dismutase activity of neutrophils: To investigate the effects of Artemia protein extract on the activity of SOD, the cells were treated with Artemia extract for 24 hrs and then the cells were collected and the pellets were homogenized by 3 times freeze-thawing. Finally, the activity of SOD was measured in supernatants (Figure 6a). It should be noted that by considering the mechanism of the SOD reaction in this method, the decrease in absorbance indicates more substrate consumption and high enzyme activity. Results indicated that, the enzyme activity in these cells was increased in response to protein extracts and increasing of concentration and also the statistical analysis of the results with One-Way ANOVA software showed that in cell treatment with crude extract and isolated fractions show significant difference at 100 µg/ml in comparison to the untreated control cells (P<0.05. In order to investigate the effect of each component and crude protein extract of Artemia urmiana on superoxide dismutase activity in human neutrophil cells after 0-24 hours of incubation, the percentage of controls were calculated (Figure 6b). 5 μg/ml of F2 fraction, 25 and 50 μg/ml of F5 and crud extract and 100 μg/ml of F3 showed more effective than other components in increasing the activity of SOD.
Discussion
Artemia Umiana is a species of aquatic crustacean, native to Lake Urmia of Iran. Artemia is a unique species due to its good adaptation to environmental stresses (including temperature, salinity and ambient oxygen content) and its ability to produce dormant cysts to live in such conditions [26]. In 2004, Choline and Clegg isolated Artemia franciscana protein P26, which protects the RNA and DNA of COS-1 cells from the damaging effects of UV light and heat [27]. Studies in mammalian cells have shown that these cells show effective resistance to the oxidative effects of oxygen free radicals (ROS) by receiving P26 and Trihalose) a sugar abundant in Artemia (although the mechanism of action is anti-inflammatory.
In the SDS-PAGE analysis, our result show that, the crude extract contains all the proteins of an organism. The pattern of F2 and F3 contain a protein band in the region of 26-27 kDa, which can be in accordance with the protein bands P26. In fractions 2, 3 and 4 a protein band in the range of 32 kDa is observed which probably belong to Artimin. All protein fractions have a protein band in the range of 65 kDa, which can be consistent with Super Oxide Dismutase (SOD) protein bands (16 kDa subunits and normal molecular weight of 65 kDa [28]. The results of SDS-PAGE gel also show that fractions 2, 3 and 4 have a higher number of protein bands and are more colorful, and fractions 6, 7 and 8 have a lower number of protein bands and less color.
The body's antioxidant defense system is comprised of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase, transition metal binding proteins such as ferritin and ceruloplasmin, and hormones such as estrogen and melatonin [29]. Other antioxidants of dietary origin include vitamins, polyphenols, and carotenoids. Oxidative stress occurs when the body's antioxidant defenses are overwhelmed. Cellular and extracellular macromolecules (i.e., proteins, lipids, and nucleic acids) can suffer oxidative damage, which may initiate or promote the development of atherosclerosis, inflammation, neoplasia, and possibly the aging process itself.
In free radical scavenger and anti-oxidant activity, despite the widespread use of 2 and 2 diphenyl-1-picryl hydrazyl (DPPH), this test in some cases gives incorrect results [30]. Therefore, if this test is used, it is better to have a reference standard for this test as an antioxidant. For this purpose, we used Ascorbic Acid as a reference standard. The results obtained from two methods of stable radical inhibition of DPPH and evaluation of regenerative power showed that the crude extract and each of the protein components and fractions of Artemia Urmiana have anti-radical activity. However, its anti-radical activity is lower compared to ascorbic acid. Comparison of anti-radical activity and reduction power of fractions shows that fractions 6 and 8 have lower IC50 than other components and therefore have higher anti-radical power as well as higher reduction power. Fractions 4 and 5 have higher IC50 than other components and therefore have lower anti-radical power as well as lower reducing powers. Fractions 2 and 3 have less anti-radical power and reduction power than fractions 6 and 8, but have more anti-radical power and reduction power than fractions 4 and 5.
Also, the results obtained from two cellular tests, namely the evaluation of NBT reduction ability and superoxide dismutase activity, showed that the crude extract and the above components may have antioxidant activity on neutrophil cells, but due to the above tests only in conditions Normal (without stimulation) results obtained have high absorption power and therefore no significant difference. However, the common results of these two tests indicate better effects of crude extract, fractions 2, 3 and 5 on neutrophil cells and lack of effect of fractions 6 and 8, which can be due to low protein content. Tom it’s. The main oxygen radicals produced in neutrophil respiration are superoxide anions (O2•-). These superoxide anions are converted to other reactive oxygen species including hydrogen peroxide (H2 O2 ), hypochlorite acid (HOCl), hydroxyl radicals (OH), and single oxygen [31]. The UVA and UVB protective ability of the total protein and its purified components will also be examined by the in-vivo methods.
Conclusion
In general, crude extract, fractions 6 and 8 showed the best anti-radical activity in the DPPH test and regenerative power, while in the cell test, these fractions showed the lowest antioxidant activity. The highest antioxidant activity in the cell test belonged to crude extract, fractions 2, 3 and 5. Therefore, based on the results obtained from this design, it can be said that the crude extract and each of the protein components of Artemia Urmiana have antioxidant compounds and also have antioxidant activity on neutrophil cells.
Declarations
Acknowledgment: This work was supported by research funds (No. 442) from National Institute of Genetic Engineering and Biotechnology, Tehran- Iran.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethical approval: The study protocol was approved by the Research Ethics Committee at National institute of Genetic Engineering and Biotechnology (Tehran-Iran).
Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Authors contributions: AD designed research, wrote the paper, conducted review and editing; PS preformed the experimental work and analysis. All authors read and approved their specific contributions.
References
- Lawson M, Jomova K, Poprac P, Kuča K, Musílek K, Valko M. Free Radicals and Antioxidants in Human Disease. In: Al-Gubory K., Laher I. (eds) Nutritional Antioxidant Therapies: Treatments and Perspectives. Springer, Cham. 2017. https://doi.org/10.1007/978-3-319-67625-8_12.
- Márcio Carocho, Isabel CFR. Ferreira, Patricia Morales, and Marina Soković Antioxidants and Prooxidants: Effects on Health and Aging, Oxidative Medicine and Cellular Longevity Volume. 2018; 2: 1472708. https://doi.org/10.1155/2018/1472708.
- Stephen Joseph McMahon and Kevin M. Prise. A Mechanistic DNA Repair and Survival Model (Medras): Applications to Intrinsic Radiosensitivity, Relative Biological Effectiveness and Dose-Rate Front. Oncol. 2021. https://doi.org/10.3389/fonc.2021.689112.
- Cesquini M, Torsoni MA, Stoppa GR, Ogo SH. T- BOOH- Induced Oxidative Damage in Sickle Red Blood Cells and the Role of Flavonoids. Biomed. Pharmacother. 2003; 57: 124-129.
- Halliwell B, Gutteridge JMC Halliwell B, Gutteridge JMC, (1989). Free radicals, ageing and disease. In: Halliwell B, Gutteridge JMC, eds. Free Radicals in Biology and Medicine. 2nd edn. Oxford: Clarendon Press. 1989: 446-493.
- Sergio Di Meo, Tanea T. Reed, Paola Venditti and Victor Manuel Victor (2016) Role of ROS and RNS Sources in Physiological and Pathological Conditions, Oxidative Medicine and Cellular Longevity Volume. 2016; 44: 1245049. http://dx.doi.org/10.1155/2016/1245049.
- Dong-Ping Xu, Ya Li, Xiao Meng, Tong Zhou, Yue Zhou, Jie Zheng, Jiao-Jiao Zhang and Hua-Bin Li, (2017) Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources, Int J Mol Sci. 2017; 18(1): 96. doi: 10.3390/ijms18010096.
- Borut Poljsak, Vito Kovaˇc and Irina Milisav, Perspective Antioxidants, Food Processing and Health, Antioxidants. 2021; 10: 433. https://doi.org/10.3390/antiox10030433.
- Wayner DD, Burton GW, Ingold KU, Barclay LR, Locke SJ. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987; 924: 408-419.
- Pyo YH, Lee TC, Logendrac L, Rosen RT, Antioxidant Activity and Phenolic Compounds of Swiss Chard (Beta Vulgaris Subspecies Cycla) Extracts. Food Chem. 2004; 85: 19-26.
- Tanguay JA, Reyes RC. & Clegg JS. Habitat diversity and adaptation to environmental stress in encysted embryos of the crustacean Artemia. J. Biosci. 2004; 29: 489-501. https://doi.org/10.1007/BF02712121.
- Thomas H. MacRae, Stress tolerance during diapause and quiescence of the brine shrimp, Artemia, Cell Stress Chaperones. 2016; 21(1): 9-18. doi: 10.1007/s12192-015-0635-7.
- Amin Eimanifar and Feridon Mohebbi, Urmia Lake (Northwest Iran): a brief review, Saline Systems. 2007; 3(5): 1-8.doi:10.1186/1746-1448-3-5.
- Theodore J. Abatzopoulos, Francisco Amat, Athanasios D. Baxevanis, Genuario Belmonte, Francisco Hontoria, Stefania Maniatsi, Salvatore Moscatello, Graziella Mura, Nickolaj V. Shadrin, Updating Geographic Distribution of Artemia urmiana Günther, 1890 (Branchiopoda: Anostraca) in Europe: An Integrated and Interdisciplinary Approach, Int. Rev of Hydrobiology. 2009; 94(5): 560-579. https://doi.org/10.1002/iroh.200911147.
- N Agh, G Van Stappen, P Bossier, H Sepehri, V Lotfi, S M. Razavi Rouhani and P. Sorgeloos (2008) Effects of Salinity on Survival, Growth, Reproductive and Life Span Characteristics of Artemia Populations from Urmia Lake and Neighboring Lagoons, Pakistan Journal of Biological Sciences. (2009); 11(2): 164-172. DOI:10.3923/pjbs.x,164,172.
- Sorgeloos, P. The use of the brine shrimp Artemia in aquaculture. . In: The brine shrimp Artemia. 1980; 3: 25-46.
- Neda Vaseli-Hagh, Abdolkhaleg Deezagi, Mahvash Khodabandeh Shahraki Anti-aging effects of the proteins from artemia extract on human fibroblasts cell proliferation and collagen expression in induced aging conditions Annals of Biotechnology. 2018; 3: 1015.
- Deezagi A, Chashnidel A, Vaseli Hagh N, Khodabandeh Shahraki M. The Effects of Purified Artemia Extract Proteins on Proliferation, Differentiation and Apoptosis of Human Leukemic HL-60 Cells. Asian Pac J Cancer Prev. 2016 1; 17(12): 5139-5145. doi: 10.22034/APJCP.2016.17.12.5139.
- Liu J, Mclennan AG. Purification and properties of GTP: GTP guanylyltransferase from encycted embryos of the brine shrimp Artemia. J of Biol Chem. 1994; 269: 11787-94.
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001; 15: 127-130.
- Zou YL. Antioxidant activity of a flavonoid rich extract of Hypericum perforatum L. in vitro. Journal of Agriculture and Food Chemistry. 2004; 52: 5032-5039.
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001; 15: 127-130.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996; 20(7): 933-56. doi: 10.1016/0891-5849(95)02227-9.
- Cheng CW, Chen LY, Chou CW, Liang JY. (2015) Investigations of riboflavin photolysis via coloured light in the nitro blue tetrazolium assay for superoxide dismutase activity. J Photochem Photobiol B. 2015; 148: 262-267. doi: 10.1016/j.jphotobiol.2015.04.028.
- Schmidt Harald HHW. and Kelm M, Determination of nitrite and nitrate by the Griess reaction, Chapter 33, In: Methods in Nitric Oxide research, edited by Martin Feelisch and Jonathan S. Stamler, 1996; 491-497.
- Granger DL, Anstey NM, Miller William C. and Weinberg J.B., Measuring nitric oxide production in human clinical studies, Methods in Enzymology. 1999; 301: 49-61.
- MacRae TH. Stress tolerance during diapause and quiescence of the brine shrimp, Artemia. Cell stress & chaperones. 2016; 21(1): 9-18. https://doi.org/10.1007/s12192-015-0635-7.
- Crista H. Collins, James S. Clegg A small heat-shock protein, p26, from the crustacean Artemia protects mammalian cells (Cos1) against oxidative damage, Cell Biology International. 2004; 28(6): 449-455. https://doi.org/10.1016/j.cellbi.2004.03.014.
- Toshiki Nakano, Minoru Sato, Masaaki Takeuchi Unique molecular properties of superoxide dismutase from teleost fish skin, FEBS Letter. 1995; 360(2): 197-201. https://doi.org/10.1016/0014-5793(95)00084-M.
- Alejandro Romero, Eva Ramos, Cristóbal de Los Ríos,Javier Egea, Javier del Pino, Russel J. Reiter, A review of metal-catalyzed molecular damage: protection by melatonin, J. of Pineal Research. 2014; 56(4): 343-370. https://doi.org/10.1111/jpi.12132.
- Om Sharma, Talapady N Bhat DPPH antioxidant assay revisited, Food Chemistry. 2009; 113(4): 1202-1205. DOI: 10.1016/j.foodchem.2008.08.008.
- Jonathan Peake and Katsuhiko Suzuki Neutrophil activation, antioxidant supplements and exercise-induced oxidative stress Running Title: Neutrophils, oxidative stress and antioxidants, Exerc Immunol Rev. 2004; 10: 129-141.