Research Article
Volume 2, Issue 7
Cost-Effectiveness Analysis of Tiotropium + Olodaterol Fixed-Dose Combination for Patients with Moderate-to-Very Severe Chronic Obstructive Pulmonary Disease in China
Yajie Gu1,2; Zhuolin Zhang3; Xin Li3,4*; Junrong Zhu1,2*
1Department of Pharmacy, Nanjing First Hospital, China Pharmaceutical University, Nanjing, China.
2Department of Pharmacy, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
3School of Pharmacy, Nanjing Medical University, Nanjing, China.
4School of Pharmacy, Nanjing Medical University, Nanjing, China. Center for Global Health, School of Public Health, Nanjing
Medical University, Nanjing, China.
Corresponding Author:
: Junrong Zhu & Xin Li
Email: junrong_zhu@aliyun.com & xinli@njmu.edu.cn
Received : Jun 29, 2023 Accepted : Jul 18, 2023 Published : Jul 25, 2023 Archived : www.meddiscoveries.org
Citation: Gu Y, Zhang Z, Li X, Zhu J. Cost-Effectiveness Analysis of Tiotropium + Olodaterol Fixed-Dose Combination for Patients with Moderate-to-Very Severe Chronic Obstructive Pulmonary Disease in China. Med Discoveries. 2023; 2(7): 1057.
Copyright: © 2023 Zhu J & Li X. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Chronic Obstructive Pulmonary Disease (COPD) is a kind of chronic progressive respiratory disease with considerable effect on patients and society. The launch of tiotropium + olodaterol Fixed-Dose Combination (FDC) provides a new choice for patients with moderateto-very severe COPD in China. The purpose of this study is to evaluate the cost-effectiveness of tiotropium + olodaterol FDC compared with tiotropium for the treatment of patients with moderate-to-very severe COPD from the perspective of the Chinese healthcare system. A Markov model was developed. The cycle length was 3 months. Patient characteristics and treatment effects from DYNAGITO and TOnado studies were entered into the model. The mortality rates from Chinese life tables were adjusted by hazard ratios. The time-to-treatment-discontinuation curve was first used to calculate the discontinuation rate of different therapies over a model cycle. Costs were obtained from official government documents and published cost-effectiveness analyses of the maintenance treatment of COPD in China. Uncertainty was assessed by one-way and probabilistic sensitivity analysis. The base case analysis reveals that tiotropium + olodaterol FDC was cost-effective, compared to tiotropium, with an incremental cost-effectiveness ratio of CNY108140.11 per quality-adjusted life year (QALY) gained. Results are robust in one-way and probabilistic sensitivity analyses. Tiotropium + olodaterol FDC is a cost-effective alternative compared to tiotropium for patients with moderate-to-very severe COPD in China under three times China’s gross domestic product (GDP) per capita threshold.
Keywords: Chronic obstructive pulmonary disease; Tiotropium + olodaterol fixed-dose combination; Cost-effectiveness analysis; Discontinuation.
Introduction
The Global initiative for chronic Obstructive Lung Disease (GOLD) strategy report defines chronic obstructive pulmonary disease (COPD) as a common treatable disease, characterized by persistent airflow limitation that is due to airway and/or alveolar abnormalities [1,2]. The persistent airflow limitation and symptoms such as shortness of breath, cough, and expectoration are typical features of COPD. The economic burden of COPD is extensive for both the individual and society. In China, COPD was the third leading cause of death [3]. In 2015, 99.9 million Chinese adults aged 20 years or older have been diagnosed with COPD [3]. The direct medical cost of COPD even reached 3565 dollars, accounting for 118.09% of the local average annual income [4]. In addition, patients with COPD tend to report the quality of life reduced [5].
Inhaled bronchodilator treatment is the backbone of maintenance therapy for COPD [6]. The results of completed clinical trials suggested that long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs) in combination could reduce the risk of exacerbation, delay the disease progression, ameliorate lung function and the symptom of dyspnea, and improve the quality of life [7-10]. Dual LAMA/LABA bronchodilator therapy is recommended for patients with severe dyspnea, airflow obstruction, and excessive inflation [2-11].
Tiotropium + olodaterol FDC is a once-daily fixed-dose dual bronchodilator that combines the LAMA tiotropium and the LABA olodaterol. This FDC has been approved for the maintenance treatment of COPD and entered the Chinese market in 2018. Tiotropium + olodaterol FDC reduced COPD exacerbation rates [12], significantly improved pulmonary function [13,14], and enhanced health-related quality of life [13]. The cost-effectiveness of tiotropium + olodaterol FDC is still unknown in China though the effectiveness and safety of that have been proven. Therefore, this study evaluated the cost-effectiveness of tiotropium + olodaterol FDC for patients with moderate-to-very severe COPD from the perspective of the Chinese healthcare system, aiming to explore whether it is worth getting tiotropium + olodaterol FDC into Chinese health insurance.
Materials and methods
Model design
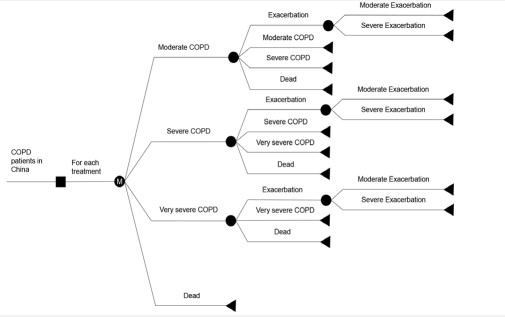
It is necessary to build a prolonged model for COPD because of the chronic and progressive features of COPD. According to the China Guidelines for Pharmacoeconomic Evaluations 2020 [15] and published literature [16,17], a Markov model was constructed to assess the lifetime cost-effectiveness of the tiotropium + olodaterol FDC in Microsoft Excel 2016. The model schematic is shown in Figure 1.
The Markov states were classified according to the severity of airflow limitation. The rationale of the classification was according to GOLD (moderate: 50% ≤ FEV1 < 80% predicted; severe: 30% ≤ FEV1 < 50% predicted; very severe: FEV1 < 30% predicted (FEV1: Forced Expiratory Volume in One Second)). The mild state was excluded from the model because moderate-tovery severe COPD is recommended to be treated with LABA/ LAMA combination by GOLD and is the indication of LABA/ LAMA combination treatment approved by the National Medical Products Administration [1,18]. Hence, only the states of moderate COPD, severe COPD, very severe COPD, and death were taken into account in the model. Meanwhile, COPD is considered irreversible [19], thus the patient in a severer state could not go back to a milder one. That is to say, a patient may stay in the current state, progress to a severer state, or enter the state of death during each cycle. A patient could enter the model from one of the three states: moderate, severe, or very severe COPD. During each cycle, patients could experience an exacerbation or remain event-free which means no exacerbation. In other words, the exacerbation was a tunnel state, which did not affect a patient’s health state. Mirror states were designed for all health states to represent the disease progression of individuals who stopped their medications. Then the patient could stay in the present state, progress into the next disease state, go into the corresponding discontinuation state or enter into the death state.
Adverse events were ignored in this study for the reason that there was no difference in the number of adverse events between the tiotropium group and the tiotropium + olodaterol FDC group in the DYNAGITO study (NCT02296138).
The analysis was conducted from a Chinese healthcare system perspective. Therefore, only direct medical costs were considered. According to the recommended intervals for follow-ups in GOLD 2023 and clinical practice in China, a model cycle length of 3 months was set which is also consistent with the DYNAGITO study. A lifetime horizon was set for the model [20-22].
Clinical inputs
Baseline characteristics
The baseline characteristics of the COPD patients were derived from the DYNAGITO study [12]. DYNAGITO study is a 52- week, randomized double-blind, phase III trial, which included Asian participants. The impact of tiotropium + olodaterol FDC 5 µg/5 µg and tiotropium 5 µg on the annual rate of exacerbations for patients with moderate-to-very severe COPD was investigated in the DYNAGITO study. Eligible patients were diagnosed with COPD, with a smoking history of more than 10 packyears and a postbronchodilator FEV1/forced vital capacity (FVC) less than 0.7, etc. The details are listed in Table 1. Accordingly, 35.62% of the patient population began in the moderate COPD state, 51.68% began in the severe COPD state, and the remaining 12.70% began in the very severe COPD state. The height and weight that were not collected in the DYNAGITO study were taken from the Report on Chinese Resident’s Chronic Disease and Nutrition 2020 [23] and the fifth bulletin on national physical fitness monitoring [24].
Comparators
Tiotropium was chosen as a comparator for tiotropium + olodaterol FDC because it is one of the recommended first-line maintenance treatments for patients with moderate-to-very severe COPD and one of the most commonly used long-acting bronchodilators for treating COPD patients in China [1,25]. In addition, tiotropium has been used as a comparator in the published economic evaluation of LABA/LAMA FDC [21,26-28]. What’s more, in DYNAGITO study and TOnado study, tiotropium was selected as a comparator [12,13]. TOnado study (Study 1237.5: NCT01431274; Study 1237.6: NCT01431287) investigated the efficacy and safety of tiotropium + olodaterol FDC compared with tiotropium or olodaterol in patients with moderateto-very severe COPD.
Transition probabilities
The transition between each Markov state of the patient is based on the method described by Spencer et al [16]. In brief, the inverse of patients’ average time in each health state was estimated as transition probabilities. The calculation formula for duration average time was reported before, which will not be mentioned here [16].
Disease progression of COPD is dependent on the annual rate of decrease in FEV1 and the initial FEV1% prediction of patients. The formula of normal people in eastern China was reported [21]:
𝐹𝐸𝑉1
= 0.04283 × 𝐻𝑒𝑖𝑔ℎ𝑡 − 0.0185 × 𝐴𝑔𝑒+ 0.39424
× 𝐺𝑒𝑛𝑑𝑒𝑟 + 0.009228832 × 𝑊𝑒𝑖𝑔ℎ𝑡 − 4.04947
Notes: Height is measured in centimeters; Weight is measured in kilograms; Gender equals 1 for males and 0 for females
The decline rate of normal people was estimated at 18.5 ml/year according to the above formula, while that of COPD patients was estimated at 42 ml/year [29]. Once the treatment starts, the improvement of trough FEV1 would be added to the baseline FEV1. Patients will progress into the next states when the FEV1 predicted reduces to the critical point of the next state. The improvement of trough FEV1 was obtained from the TOnado study, and the values were listed in Table 2.
COPD exacerbation
COPD exacerbations have a negative impact on health states, rates of hospitalization and re-admission, and disease progression, which makes COPD exacerbations non-negligible in the management of COPD. A COPD exacerbation is defined as an acute worsening event of respiratory symptoms that requires additional treatment [1,12]. An important goal in the treatment of COPD is to reduce the risk of exacerbation. The risk of exacerbations increases with the progression of disease symptoms and the deterioration of lung function.
According to GOLD 2023, COPD exacerbations are categorized as mild (treated with short-acting bronchodilators only), moderate (treated with short-acting bronchodilators plus antibiotics and/or oral corticosteroids), and severe (patients require hospitalization or emergency care). Mild exacerbation was ignored in this model due to its low incidence and minor influence on healthcare costs and health outcomes [30]. In accordance with the DYNAGITO study [12], a moderate exacerbation was defined as those leading to oral corticosteroids or antibiotics or both treatments and severe exacerbation was defined as those requiring hospitalization or an emergency room visit.
The incidence of moderate and severe exacerbation per cycle was calculated based on the rate of different exacerbation events during the year in the DYNAGITO study.
Discontinuation rate
Discontinuation rates were retrieved from the DYNAGITO study, by picking up points in the time-to-treatment-discontinuation curve in GetData Graph Digitizer [12]. Assuming patients receive medication guidance from clinical pharmacists every three months (a cycle) in every follow-up, the discontinuation rate will go back to the initial level after each follow-up. For the convenience of calculation, the mean discontinuation rate of the first 90 days was taken into calculation. Patients may discontinue their treatment due to poor efficacy and/or the occurrence of intolerable adverse reactions and/or poor adherence. No matter what reason for discontinuation, only the impact after discontinuation will be taken into account. The clinical impact of drug discontinuation was reflected in the input of clinical efficacy parameters. Patients who discontinued therapy will no longer benefit from improvements in trough FEV1 and thus had a higher probability of disease progression and a higher risk of exacerbation. Exacerbation rates after discontinuation were adjusted by the relative risk for tiotropium versus placebo (0.86, 95% CI: 0.81-0.91) in the UPLIFT study [29]. Patients who discontinued their treatment with inhaled medications were assumed to continue maintenance therapy.
Mortality
Death may be the result of severe exacerbation or a cause related to the severity of COPD. The mortality rate resulting from severe exacerbation was 1.24%, according to a multicenter, retrospective, observational study conducted in China [31]. The mortality related to the severity of COPD was calculated by age and sex all-cause mortality lifetable [32] and mortality risk ratios for different disease states, which were derived from previous studies [33]. To avoid double counting mortality due to severe exacerbations, the hazard ratio of 0.7 mentioned in the previous study was used to adjust for all-cause mortality [21].
Cost and utility inputs
Cost: From the perspective of the Chinese healthcare system, only direct medical costs are included. Therefore, only drug acquisition costs, maintenance costs stratified by COPD severity and exacerbation management costs were taken into account. Adverse management costs were ignored because of the non-significant difference between the two treatments in the DYNAGITO study.
The costs of tiotropium and tiotropium + olodaterol FDC were derived from the public resource trading platform of Jiangsu Province [34]. The costs of drug acquisition in a cycle were calculated by unit price and quantity. Maintenance costs which contained outpatient visits, spirometry, chest imaging examinations and influenza vaccinations were obtained from a previous expert survey [22]. Moderate exacerbation management costs included drug cost, test costs and outpatient visit costs. Severe exacerbation management costs included drug cost, test costs, nursing costs and inpatient ward costs. Exacerbation management costs were also obtained from the previous expert survey [22]. And all costs were inflated to 2021 CNY using the Chinese Consumer Price Index for Healthcare [35]. Both QALYs and costs were discounted at a rate of 5% in the base case analysis, according to the China Guidelines for Pharmacoeconomic Evaluations 2020 [15].
Utilities: The utility values for each Markov state were obtained from a Chinese health-related quality-of-life study published in 2015 [36]. The annual utility decrements were 0.010 and 0.042 for the moderate exacerbation state and severe exacerbation state [37].
Scenario and sensitivity analyses
As a lifetime horizon was performed in the base case analysis, lifetime costs and effects were discussed. In order to investigate the short-term cost-effectiveness of tiotropium + olodaterol FDC compared with tiotropium, a five-year time horizon scenario analysis performed.
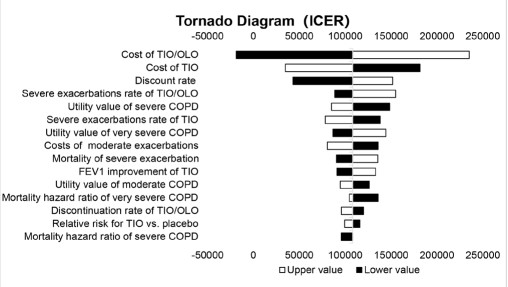
A one-way sensitivity analysis (OWSA) was conducted to test the robustness of the base case results. In OWSA, only one specific parameter changes in a test. The OWSA results for each input were ranked from the most sensitive to the least sensitive, and the tornado diagram showed the top ten parameters that had the greatest impact on the final result. The range of the improvements in FEV1 was retrieved from the previous studies [13]. The upper and lower limits of exacerbation rate [12], the relative risk for Tiotropium versus placebo [29], and the hazard ratio of all-cause mortality rate [21] were retrieved from 95% confidence intervals of published literature. The discount rate varied between 0% and 8% per annum, according to the China Guidelines for Pharmacoeconomic Evaluations 2020 [15]. Other parameters fluctuated ±20% around the base case value in OWSA. The specific values of the parameters used in OWSA are shown in Table 2.
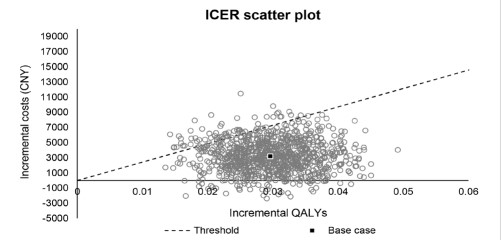
In addition, a Probabilistic Sensitivity Analysis (PSA) was conducted to estimate the uncertainty associated with costs and health outcomes. In the PSA, all parameters varied in ranges at the same time. 1000 times sampling and calculation were performed, and the results were presented in a scatter plot. In the scatter plot, each dot represented a result of once different input. The normal distribution is used for FEV1 improvement and utility decrements [21]. Log-normal distribution was applied to the relative risk for tiotropium vs. placebo and hazard ratios. Beta distribution was used for exacerbation rate, discontinuation rate, mortality of severe exacerbation, and utility. Gamma distribution was applied for costs. The 1000-times Monta Carlo simulation was performed for PSA.
Model output
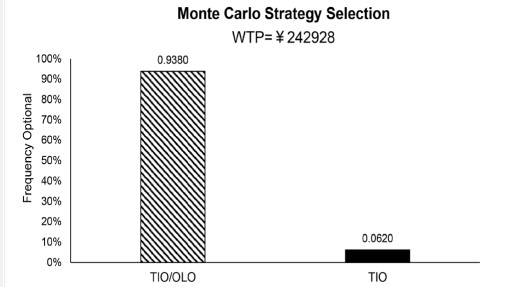
Model outputs include total costs, lifeyears, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). To calculate the cost-effectiveness of two different COPD maintenance treatments, ICER is calculated by dividing the incremental cost of the two intervention strategies by the incremental effectiveness. In this study, the willingness-to-pay (WTP) threshold is set to three times China’s (GDP) per capita in 2021 [15], i.e., CNY 242928 [35]. The tiotropium + olodaterol FDC can be considered a more cost-effective strategy for Chinese patients with moderate-to-very severe COPD compared with tiotropium when the ICER is lower than the WTP threshold.
Results
Base case analyses
Base-case analyses, using best estimates for each input variable, were performed first. As shown in Table 3, tiotropium + olodaterol FDC gains additional 0.0846 life-years and additional 0.0296 QALYs compared to tiotropium with extra CNY 3201.50 costs. As a result, the ICER is CNY 108140.11 per QALY gained, which is lower than the WTP threshold. This results in tiotropium + olodaterol FDC being a more cost-effective strategy.
Scenario and sensitivity analyses
In the scenario analysis (Table 3), tiotropium + olodaterol FDC gained extra 0.0397 lifeyears. Tiotropium + olodaterol FDC produced an incremental effectiveness of 0.0208 QALYs with an additional cost of CNY3463.69. As a result, the ICER of tiotropium + olodaterol FDC versus tiotropium was CNY166455.45 per QALY, which was still lower than the WTP. However, compared to base case analysis, it seems that COPD patients will benefit more from tiotropium + olodaterol FDC in the long run.
The results of the OSWA are shown in the tornado diagram in Figure 2. The parameters that have the greatest impact on the results are the cost of tiotropium + olodaterol FDC, the cost of tiotropium and the discontinuation rate. However, the result of the OSWA is relatively stable. When the cost of tiotropium + olodaterol FDC is at its highest value, the of ICER is CNY235082, which is still lower than the WTP. What’s more, the ICER is negative when the cost of tiotropium + olodaterol FDC is at its lowest value, which indicates tiotropium + olodaterol FDC is a dominant alternative. In conclusion, tiotropium + olodaterol FDC is a more cost-effective strategy compared to tiotropium under the WTP threshold, though the parameters have varied within a set range.
The results of the PSA are shown in Figure 3. Tiotropium + olodaterol FDC results in an increase in QALYs versus tiotropium in all simulations, and tiotropium + olodaterol FDC is more costeffective than tiotropium in 93.8% of simulations (Figure 4).
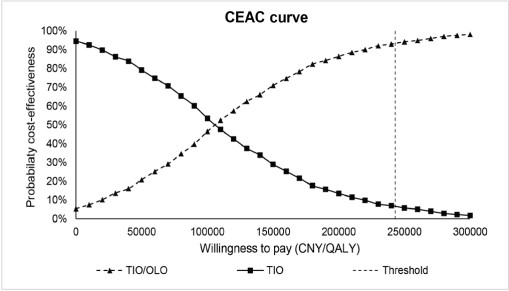
The cost-effectiveness acceptability curve (Figure 5) indicates that tiotropium + olodaterol FDC has a probability over 98% of being cost-effective when the WTP threshold is over CNY 300000.
Table 1: Baseline characteristics of the target population.
| Baseline characteristics | value | Reference |
|---|---|---|
| Age | 66.5 | 12 |
| Gender (male) | 71.00% | 12 |
| Height (male, cm) | 169.7 | 23 |
| Height (Female, cm) | 158.0 | 23 |
| Weight (male, kg) | 67.35 | 24 |
| Weight (female, kg) | 59.23 | 24 |
| Initial distribution of disease state | ||
| Moderate COPD | 35.62% | 12 |
| Severe COPD | 51.68% | 12 |
| Very severe COPD | 12.70% | 12 |
Abbreviations: cm: centimeter; kg: kilometer; COPD: Chronic Obstructive Pulmonary Disease; FDC: Fixed-Dose Combination; QALY: QualityAdjusted Life-Year.
Table 2: Model inputs for base-case and sensitivity analyses.
| Parameters | Base-case value | OWSA value | Distribution | Source | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Trough FEV1 improvement | |||||
| TIO/OLO | 0.1405 | 0.1360 | 0.1450 | Normal | 13 |
| TIO | 0.0805 | 0.0650 | 0.0950 | Normal | 13 |
| Hazard ratio of all-cause mortality rate | |||||
| Moderate COPD | 1.466 | 1.19 | 1.48 | Log-normal | 33 |
| Severe COPD | 3.025 | 1.97 | 2.97 | Log-normal | 33 |
| Very severe COPD | 5.256 | 2.75 | 5.76 | Log-normal | 33 |
| Exacerbations rate | |||||
| TIO/OLO | |||||
| Moderate | 0.2364 | 0.2149 | 0.2538 | Beta | 12 |
| Severe | 0.0663 | 0.0602 | 0.0757 | Beta | 12 |
| TIO | |||||
| Moderate | 0.2599 | 0.2308 | 0.2792 | Beta | 12 |
| Severe | 0.0757 | 0.0694 | 0.0853 | Beta | 12 |
| Relative Risk for Tiotropium vs. Placebo | 0.86 | 0.81 | 0.91 | Log-normal | 29 |
| Mortality rate of severe exacerbation | 0.0124 | 0.0112 | 0.0136 | Beta | 29 |
| Discontinuation rate | |||||
| TIO/OLO | 0.0207 | 0.0167 | 0.0248 | Beta | 12 |
| TIO | 0.0740 | 0.0369 | 0.0553 | Beta | 12 |
| Cost (per cycle) | |||||
| Drug acquisition | |||||
| TIO/OLO | 2168.76 | 1735.01 | 2602.51 | Gamma | 38,39 |
| TIO | 1560.00 | 1248.00 | 1872.00 | Gamma | 38,39 |
| Maintenance | |||||
| Moderate COPD |
1547.87 | 1238.29 | 1857.44 | Gamma | 21 |
| Severe COPD |
2109.30 | 1687.44 | 2531.16 | Gamma | 21 |
| Very severe COPD | 3189.41 | 2551.53 | 3827.29 | Gamma | 21 |
| Exacerbation management (per episode) | |||||
| Moderate exacerbation | 2056.19 | 1644.95 | 2467.43 | Gamma | 21 |
| Severe exacerbation | 18875.2 | 15100.16 | 22650.24 | Gamma | 21 |
| Utility | |||||
| Moderate COPD | 0.734 | 0.587 | 0.881 | Beta | 36 |
| Severe COPD | 0.691 | 0.553 | 0.829 | Beta | 36 |
| Very severe COPD | 0.675 | 0.540 | 0.810 | Beta | 36 |
| Moderate exacerbation | 0.010 | 0.008 | 0.012 | Normal | 21,37 |
| Severe exacerbation | 0.042 | 0.034 | 0.050 | Normal | 21,37 |
Notes: All costs are presented in CNY (2021).
Abbreviations: OWSA: One-Way Sensitivity; FEV1: Forced Expiratory Volume In One Second; TIO/OLO: Tiotropium + Olodaterol
Fixed-Dose Combination; TIO: Tiotropium, COPD: Chronic Obstructive Pulmonary Disease; QALY: Quality-Adjusted Life-Year.
Table 3: Base case and Scenario analyses results.
| Base case analysis | Scenario analysis | |||||
|---|---|---|---|---|---|---|
| TIO/OLO | TIO | difference | TIO/OLO | TIO | difference | |
| LYs | 18.7782 | 18.6936 | 0.0846 | 14.1567 | 14.1170 | 0.0397 |
| Costs (CNY) | 66065.55 | 62864.04 | 3201.50 | 58747.33 | 55.283.65 | 3463.69 |
| QALYs | 7.2995 | 7.2699 | 0.0296 | 6.5073 | 6.4865 | 0.0208 |
| ICER, CNY/ QALY | 108140.11 | 166455.45 | ||||
Notes: All costs are presented in CNY (2021).
Abbreviations: TIO/OLO: Tiotropium + Olodaterol Fixed-Dose Combination; TIO: Tiotropium; Lys: Lifeyears; FDC: Fixed-Dose Combination;
QALY: Quality-Adjusted Life-Year; ICER: Incremental Cost-Effectiveness
Ratios.
Discussion
This analysis is the first to investigate the cost-effectiveness of tiotropium + olodaterol FDC and tiotropium for Chinese patients with moderate-to-very severe COPD. The result revealed that tiotropium + olodaterol FDC was more cost-effective for patients with moderate-to-very severe COPD than tiotropium from the Chinese healthcare system perspective, which reduced the risk of exacerbation and improved the quality of life. Scenario analysis revealed that tiotropium + olodaterol FDC was still an economic alternative over five years, notwithstanding a higher ICER than that of base case analysis. In the OWSA, the costs of tiotropium + olodaterol FDC and tiotropium had the greatest impact on the results. The model used in this study was based on previously reported models [16,17] and adjusted based on the Chinese healthcare system. This study was conducted by the China Guidelines for Pharmacoeconomic Evaluations 2020. We first made use of the time-to-treatment-discontinuation curve to calculate the discontinuation rate over three months.
In the TOnado study, tiotropium + olodaterol FDC was shown to improve lung function and quality of life and reduce dyspnea compared with tiotropium [13]. The DYNAGITO study found that the tiotropium + olodaterol FDC reduced the annual rate of total exacerbations (P<0.05) in comparison with tiotropium, and has no significant difference in safety [12].
In terms of pharmacoeconomic evaluation, Selya-Hammer et al [38] evaluated the cost-effectiveness of tiotropium + olodaterol FDC and tiotropium from the perspective of the Italian National Health Service and the result showed that tiotropium + olodaterol FDC was a more economical option compared to tiotropium. van Boven et al [26] conducted a cost-versus-effectiveness analysis of tiotropium + olodaterol FDC and tiotropium from the perspective of Dutch healthcare payers and the results similarly showed that tiotropium + olodaterol FDC was a costeffective therapeutic regimen compared to tiotropium. All results are consistent with the results of this study.
Several economic evaluations of dual LAMA/LABA bronchodilators have been conducted in China [21,39]. However, the lack of head-to-head randomized clinical trials of available dual LAMA/LABA bronchodilators limits the cost-effective assessment of all available dual LAMA/LABA bronchodilators based on direct high-quality evidence. Further head-to-head randomized clinical trials need to be carried out.
This study also has limitations due to data and methodological deficiencies. Populations of different ethnic backgrounds may exhibit variability in response to tiotropium + olodaterol FDC. Though the clinical on which this study is based included 14.77% of Asians, individual data were not available for a subgroup of the Chinese population and the subgroup analysis was not performed. Therefore, the results may be inaccurate. However, according to a published study [40], tiotropium + olodaterol FDC is more effective in the Chinese population relative to the overall population, with similar safety. Therefore, it is reasonable to suppose that tiotropium + olodaterol FDC would be more cost-effective in the Chinese population. Similar to other cost-effectiveness analyses based on clinical trials, the results may be different in the real world because of the strict inclusion and exclusion criteria and process management of the clinical trial.
The control drug used in the DYNAGITO study was tiotropium bromide inhalation spray, therefore, tiotropium bromide inhalation spray was also used as the comparator in this analysis. There are two dosage forms of tiotropium inhalation formulations in China, one is tiotropium bromide inhalation spray (2.5 μg, 60 sprays, two sprays, QD) and the other is tiotropium bromide powder for inhalation (18 μg/capsule, one capsule, QD). Among them, a generic version of tiotropium bromide spray is not yet available in China and therefore tiotropium bromide inhalation spray has a higher price, while tiotropium bromide powder has been marketed in China in a variety of generics. It has been shown that the two different dosage forms of tiotropium inhalation formulations are bioequivalent [41]. However, only the cost of tiotropium bromide inhalation spray was considered in the study because the market share of these two dosage forms in China was not available, and tiotropium bromide inhalation spray is used in randomized controlled trials [12,13].
Conclusion
To sum up, from the perspective of the Chinese healthcare system, tiotropium + olodaterol FDC could be considered as a cost-effective alternative of maintenance treatment for moderate-to-very severe COPD patients, when compared to tiotropium. This economic evaluation of tiotropium + olodaterol FDC may provide policy basis for health authorities.
Declarations
Funding: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflicts of interest: All authors declare that they have no relevant financial or non-financial interests.
Availability of data and material: All data generated or analyzed during this study are included in this published article.
Author contributions: All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yajie Gu and Zhuolin Zhang. The first draft of the manuscript was written by Yajie Gu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 2023 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. 2022.
- Chronic Obstructive Pulmonary Disease Group of the Chinese Medical Association, Respiratory Diseases Branch, Chronic obstructive pulmonary disease working committee of respiratory physician branch of Chinese Medical Association. Guidelines for the management of chronic obstructive pulmonary disease (2021 revised edition). Chinese Journal of Tuberculosis and Respiratory Diseases. 2021; 44: 170-205.
- Wang C, Xu J, Yang L, Xu Y, Zhang X, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. The Lancet. 2018; 391: 1706-1717.
- Zhu B, Wang Y, Ming J, Chen W, Zhang L. Disease burden of COPD in China: a systematic review. COPD. 2018; 13: 1353-1364.
- Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002; 19: 217-224.
- Chinese Medical Doctor Association Branch of Respiratory Doctors, Respiratory Disease Branch of Chinese Medical Association, Respiratory Rehabilitation Committee of China Rehabilitation Medical Association, Editorial Committee of Chinese Journal of Health Management. Chinese Guidelines for Respiratory Rehabilitation Management of Chronic Respiratory Diseases. Chinese Journal of Health Management. 2021; 15: 521-538.
- Ray R, Tombs L, Naya I, Compton C, Lipson DA, et al. Efficacy and safety of the dual bronchodilator combination umeclidinium/vilanterol in COPD by age and airflow limitation severity: A pooled post hoc analysis of seven clinical trials. Pulm Pharmacol Ther. 2019; 57: 101802.
- Maltais F, Bjermer L, Kerwin EM, Jones W, Watkins Michael L, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: The EMAX randomised trial. Respir Res. 2019; 20: 238.
- Cazzola M, Molimard M. The scientific rationale for combining long-acting beta(2)-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010; 23: 257-267.
- Martinez FJ, Fabbri LM, Ferguson GT, Orevillo C, Darken P, et al. Baseline Symptom Score Impact on Benefits of Glycopyrrolate/ Formoterol Metered Dose Inhaler in COPD. Chest. 2017; 152: 1169-1178.
- Gong Y, Lv Y, Liu H, Zheng Q, Li L. Quantitative analysis of efficacy and safety of LABA/LAMA fixed-dose combinations in the treatment of stable COPD. Therapeutic Advances in Respiratory Disease. 2022; 16: 1-14.
- Calverley PMA, Anzueto AR, Carter K, Grönke L, Hallmann C, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. The Lancet Respiratory Medicine. 2018; 6: 337-344.
- Buhl R, Maltais F, Abrahams R, Bjermer L, Derom E, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4). Eur Respir J. 2015; 45: 969-979.
- Mahler DA, Ludwig-Sengpiel A, Ferguson GT, de la Hoz A, Ritz J, et al. TRONARTO: A Randomized, Placebo-Controlled Study of Tiotropium/Olodaterol Delivered via Soft Mist Inhaler in COPD Patients Stratified by Peak Inspiratory Flow. COPD. 2021; 16: 2455-2465.
- Liu GE. China guidelines for pharmacoeconomic evaluations 2020. 1st edition. Beijing: China Market Press. 2020.
- Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005; 23: 619-637.
- Oostenbrink JB, Rutten-van Mölken MPMH, Monz BU, FitzGerald JM. Probabilistic Markov Model to Assess the Cost-Effectiveness of Bronchodilator Therapy in COPD Patients in Different Countries. Value in Health. 2005; 8: 32-46.
- National basic medical insurance, industrial injury insurance and Birth Insurance Drug List. 2021.
- Buttery SC, Zysman M, Vikjord SAA, Hopkinson NS, Jenkins C, Vanfleteren LEGW. Contemporary perspectives in COPD: Patient burden, the role of gender and trajectories of multimorbidity. Respirology. 2021; 26: 419-441.
- Zhou Y, Long E, Xu Q, Wang L, Jiang X, et al. Cost-Effectiveness Analysis of Triple Combination Preparations in the Treatment of Moderate-to-Severe Chronic Obstructive Pulmonary Disease. Front Public Health. 2021; 9: 713258.
- Wang L, Gu W, Zhang X, Fu S, Zhang D, et al. How the cost-effectiveness results change in the China health policy environment: an economic evaluation of glycopyrrolate/formoterol for the treatment of COPD. Journal of Medical Economics. 2022; 25: 356-366.
- Liu J, He X, Wu J. Economic Evaluation of Triple Therapy with Budesonide/Glycopyrrolate/Formoterol Fumarate for the Treatment of Moderate to Very Severe Chronic Obstructive Pulmonary Disease in China Using a Semi-Markov Model. Appl Health Econ Health Policy. 2022; 20: 743-755.
- Report on Chinese Resident’s Chronic Disease and Nutrition 2020. Accessed Jul 18, 2022.
- The Fifth Bulletin on National Physical Fitness Monitoring. 2022.
- Li D, Li S. Comparison and Analysis of Market Structure and Size of Orally Inhaled Drug Products for Treatment of Asthma and COPD. Chinese Journal of Pharmaceuticals. 2020; 51: 1602–10.
- van Boven J, Kocks J, Postma MJ. Cost-effectiveness and budget impact of the fixed-dose dual bronchodilator combination tiotropium-olodaterol for patients with COPD in the Netherlands. COPD. 2016; 11: 2191-2201.
- Hoogendoorn M, Corro Ramos I, Soulard S, Cook J, Soini E, et al. Cost-effectiveness of the fixed-dose combination tiotropium/ olodaterol versus tiotropium monotherapy or a fixed-dose combination of long-acting β2-agonist/inhaled corticosteroid for COPD in Finland, Sweden and the Netherlands: A model-based study. BMJ Open. 2021; 11: e049675.
- Chan MC, Tan ECH, Yang MC. Cost-effectiveness analysis of a fixed-dose combination of indacaterol and glycopyrronium as maintenance treatment for COPD. COPD. 2018; 13: 1079-1088.
- Tashkin D, Celli B, Senn S, Burkhart D, Kesten S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease (UPLIFT trial). Rev Port Pneumol. 2009; 15: 137-140.
- Hanania NA, Tashkin DP, Kerwin EM, Donohue J, Denenberg M, et al. Long-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co-SuspensionTM Delivery Technology in patients with chronic obstructive pulmonary disease. Respiratory Medicine. 2017; 126: 105-115.
- Zhang J, Zheng J, Huang K, Chen Y, Yang J, et al. Use of glucocorticoids in patients with COPD exacerbations in China: a retrospective observational study. Ther Adv Respir Dis. 2018; 12: 175346661876951.
- China Population Census Yearbook. 2020.
- Ekberg-Aronsson M, Pehrsson K, Nilsson JÅ, Nilsson PM, Löfdahl CG. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res. 2005; 6: 98.
- Drug Consumables Procurement. Jiangsu Public Resources Trading Center. 2022.
- National data. 2022.
- Wu M, Zhao Q, Chen Y, Fu C, Xu B. Quality of life and its association with direct medical costs for COPD in urban China. Health Qual Life Outcomes. 2015; 13: 57.
- Samyshkin Y, Schlunegger, Haefliger, Ledderhose, Radford. Costeffectiveness of roflumilast in combination with bronchodilator therapies in patients with severe and very severe COPD in Switzerland. COPD. Published online January. 2013:79.
- Selya-Hammer C, Gonzalez-Rojas Guix N, Baldwin M, Ternouth A, Miravitlles M, et al. Development of an enhanced healtheconomic model and cost-effectiveness analysis of tiotropium + olodaterol Respimat ® fixed-dose combination for chronic obstructive pulmonary disease patients in Italy. Ther Adv Respir Dis. 2016; 10: 391-401.
- Gong S, Hu H, Zhao K, Yang T. Cost-Effectiveness of Dual Bronchodilator Indacaterol/Glycopyrronium for COPD Treatment in China. COPD. 2021; 16: 433-441.
- Bai Chunxue, Tang Yan, Xin Jianbao, Li Yali, Li Zhikui, et al. The efficacy and safety of tiotropium / olodaterol fixeddose combination in Chinese patients with chronic obstructive pulmonary disease: a pooled subgroup analysis of TONADO 1+2. Chinese Journal of Tuberculosis and Respiratory Diseases. 2019; (11):838-839-840-841-842-843-844.
- Algorta J, Andrade L, Medina M, Kirkov V, Arsova S, et al. Pharmacokinetic Bioequivalence of Two Inhaled Tiotropium Bromide Formulations in Healthy Volunteers. Clin Drug Investig. 2016; 36: 753-762.