Case Report
Volume 2, Issue 7
Statin Therapy and Tendon Rupture in High ASCVD Score Patient: A Comprehensive Case Report and Literature Review
Mahsa Fadaei1*; Mehrdad Jafari Tadi2; Seyyed Taher Seyyed Mahmoudi3; Parisa Kianpour4; Sina Ghobadi5; Mohommad Rasekhi Siahkal Mahalleh6; Shima Rafie7; Seyyed Mahdi Seyyed Mahmoudi8; Reza Mourtami9
1Ahvaz Medical School Research committee, Ahvaz, Iran.
2Research Committee, Shahid Beheshti University of Medical Science, Iran.
3School of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
4Clinical Pharmacy Department, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
5Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
6School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
7Tehran University of Medical Sciences, Iran.
8School of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
9Tehran University of Medical Sciences School of Medicine, Iran.
Corresponding Author:
Mahsa Fadaei
Email: dr.mahsafadaei@gmail.com
Received : Jun 18, 2023 Accepted : Jul 12, 2023 Published : Jul 19, 2023 Archived : www.meddiscoveries.org
Citation: Fadaei M, Mehrdad Jafari T, Seyyed Taher SM, Kianpour P, Ghobadi S, et al. Statin Therapy and Tendon Rupture in High ASCVD Score Patient: A Comprehensive Case Report and Literature Review. Med Discoveries. 2023; 2(7): 1055.
Copyright: © 2023 Fadaei M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Statin-induced tendon rupture is a rare clinical manifestation that warrants attention, especially in patients with a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk score. This case report presents the unique scenario of a 58-year-old male with a history of ischemic heart disease and previous percutaneous coronary intervention who experienced tendon rupture following minor traumas while on long-term statin therapy. The patient underwent surgical intervention involving tendon repair to address the rupture.
Given the association between statins and tendon ruptures documented in the literature, we conducted a thorough review to identify reliable substitute medications for statins in high-risk patients with ASCVD. The literature review explored alternative treatment options that effectively manage cardiovascular risks while minimizing the potential risk of tendon rupture. The findings from this case report and literature review provide valuable insights into managing statin-induced tendon rupture and highlight the importance of considering alternative medications in patients with high 10-year ASCVD scores.
Introduction
The use of diagnostic biomarkers to diagnose common diseases is rising [1-3]. The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors or statins are used in the primary and secondary prevention of cardiovascular events [4,5].
Statins are usually well-tolerated and do not have many serious side effects. One of the side effects that can affect the decision to continue treatment with statins is musculoskeletal complications such as tendinopathy, which is most common with Atorvastatin and Simvastatin [6].
Statin-related musculoskeletal adverse effects (AE) vary from mild myalgia and muscle weakness to tendinopathy and rhabdomyolysis [1]. The probable risk factors are summarized in Table 1. Tendinopathy is usually presented with tendinitis and tendon rupture, especially of the Achilles, quadriceps, and distal biceps tendons, which mostly happen within the first year of initiation and improve after discontinuation [3].
This case report describes a 58-year-old man who experienced tendon rupture as an adverse effect of statin use. Given the significance of this complication, it is crucial to explore alternative treatment options for high-risk patients, particularly those with a high ASCVD score. By examining the existing literature, we aim to identify a reliable substitute for statins in such patients, ensuring effective management of their atherosclerotic cardiovascular disease while minimizing the risk of tendonrelated adverse events.
Case report
Patient presentation
A 58-year-old male, employed in a municipal position with a history of athletic experience, presented with two tendon ruptures in the rotator cuff, occurring in different shoulders after experiencing two minor traumas. His medical history included ischemic heart disease for which he underwent Percutaneous Coronary Intervention (PCI) four years ago. Additionally, he had a 10-year Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 22.7% and had been diagnosed with benign prostatic hypertrophy, hypertension, and diabetes mellitus. The patient’s prescribed medications consisted of 40 mg atorvastatin once daily, 0.4 mg tamsulosin once daily, 5 mg amlodipine once daily, dutasteride 0.5 mg once daily, metoprolol 12.5 mg every 12 hours, metformin 500 mg every 8 hours, pioglitazone 15 mg daily, and ASA 80 mg once daily. Notably, the patient did not consume alcohol and did not smoke.
Clinical course
Before the occurrence of these tendon ruptures, the patient reported experiencing shoulder pain following sports activities, such as soccer, which had not been a previous issue. The initial trauma occurred in 2020 while the patient was climbing a mountain with a slight slope. During this incident, he slipped on his left hand, experiencing mild pain at the moment. However, the pain was not severe enough to prompt him to seek medical attention, and similar occurrences in the past had resolved spontaneously. Subsequently, after some time (exact duration not recalled), the patient experienced a similar falling accident while participating in an indoor soccer game. This incident resulted in severe shoulder pain, leading him to consult a physician. The pain was intense, and he faced difficulties in lifting his hand.
Investigations and diagnosis
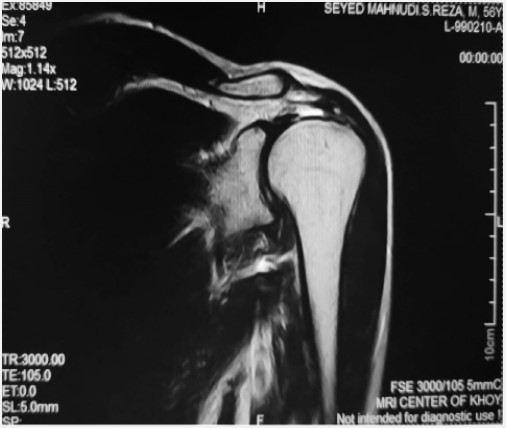
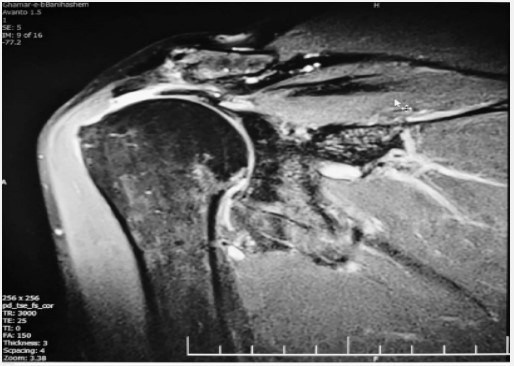
An MRI (Figure 1) revealed a full-thickness tear at the critical zone of the supraspinatus tendon, attributed to the initial mild trauma. Additionally, a partial tear of the right rotator cuff tendon was evident but remained undiagnosed and untreated at that time. After a two-year interval, the patient experienced another fall, causing the same severe, non-radiating pain that worsened during nighttime and hand abduction. A subsequent MRI (Figure 2) identified a complete rotator cuff tendon and joint effusion tear. As a result, the patient underwent a second arthroscopic tendon repair procedure.
Implications and management
Following the occurrence of these two minor traumas, the possibility of atorvastatin side effects as the underlying cause was considered. Employing the Naranjo Adverse Drug Reaction (ADR) probability scale, the patient scored a 7, indicating a probable association between atorvastatin and tendon ruptures. Consequently, atorvastatin was discontinued, and an alternative drug was sought to manage the patient’s high 10-year ASCVD risk.
Considering the necessity of reducing cardiovascular mortality while avoiding statins, evolocumab, a PCSK9 inhibitor, was selected as the suitable alternative after a comprehensive evaluation. The patient was prescribed a dose of 140 mg once every two weeks.
Table 1: Major risk factors of statin-induced musculoskeletal injury.
| Age>80 y/o |
| Female sex |
| Chronic kidney disease |
| Acute or chronic liver disease |
| Hypothyroidism |
| Recent major trauma or surgery |
| Drug interactions (ie. Fibrates, calcium channel blockers, amiodarone, pioglitazone and rosiglitazone, azole antifungals, protease inhibitors, etc.) |
Discussion
The clinical benefits of statins in reducing mortality among patients with a history of cardiovascular events are attributed to their pleiotropic effects [7-9]. Several medications have been associated with an increased risk of tendon rupture. These include:
1. Fluoroquinolones, particularly ciprofloxacin and levofloxacin, have been associated with an increased risk of tendon rupture, especially in the Achilles tendon [10].
2. Corticosteroids such as prednisone and cortisone can weaken tendons and increase the risk of rupture [11].
3. Fluconazole: Also has been associated with an increased risk of Achilles tendon rupture [12].
4. Isotretinoin toxicity: Tendinopathy also have been reported in patients taking isotretinoin [13].
5. statins: Atorvastatin and simvastatin have also been linked to an increased risk of tendon ruptures.
Although statins have been associated with an increased risk of Achilles tendon rupture, the risk is relatively low. Recent studies found that the risk of tendon rupture in statin users was 0.4%, compared with 0.2% in nonusers. The studies also found that the risk was highest in those who took high doses of statins over a long period [14]. But in addition to statin use, other risk factors, as mentioned in Table 1, may also increase the likelihood of tendon rupture, and in patients, such as those discussed in our case report, who do not have any of the risk factors listed in the table, we should consider the higher likelihood of tendon rupture due to statins [15-19].
The clinical effect of statins in reducing mortality in patients with a history of cardiovascular events is related to their pleiotropic effects, which means that, in addition to the cholesterollowering effect, they have additional effects that are responsible for the clinical benefits in patients with CVD. These effects include improvement of myocardial perfusion and reduction of recurrent anginal episodes after acute chronic events through modulation of endothelial function, plaque stabilization, neovascularization, attenuation of atherogenesis, improvement of neurohormonal imbalance, reduction of oxidative stress, vascular inflammation, and antithrombotic effects action [7,20,21].
Suggesting an alternative drug in patients requiring secondary prophylaxis for cardiovascular events is considered a serious challenge, especially in the case of adverse events such as recurrent tendinopathy requiring discontinuation of the statin.
Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9 inhibitors) are one of the hypolipidemic classes approved by the Food and Drug Administration (FDA) for treating autosomal familial hypercholesterolemia. Evolocumab, alirocumab, and cocizumab are known PCSK9 inhibitors. Their main mechanism of action is to increase LDL receptors on hepatocytes by inhibiting PCSK9, a protein responsible for suppressing LDL receptor activity [22,23].
The pleiotropic effects of this family include lipid-lowering effects, inhibition of atherogenesis, stabilization of atherosclerotic plaque, anti-inflammatory effects by increasing the concentration of interleukin 10 (IL -10) and decreasing the concentration of interleukin-1ɑ (IL -1ɑ), interleukin-6 (IL -6) and tumor necrosis factor ɑ (TNF-ɑ) [24,25], anti-aggregation and anticoagulant properties [22,26,27].
Because of its effect on reducing cardiovascular events and all-cause mortality in patients at very high risk for atherosclerotic cardiovascular disease (ASCVD) [28,29], they can be considered as a suitable alternative in patients with statin intolerance.
There are few studies on the association between PCSK9 inhibitors and tendon rupture. However, some studies suggest that PCSK9 inhibitors may negatively affect tendon health and increase the risk of tendon rupture. This is because PCSK9 inhibitors can cause a decrease in cholesterol levels, which can affect tendon structure and function [30,31].
In cases of rare but urgent conditions similar to the occurrence of statin-induced tendon rupture in patients on long-term statin therapy, it is crucial to consider alternative treatment options and prioritize anticipatory care planning and follow-up to potentially restore normal tissue function and preserve organ integrity [32-34].
Conclusion
This case highlights the significance of vigilance regarding rare yet severe adverse effects of medications, such as tendon ruptures in patients receiving statin therapy. Additionally, it underscores the importance of considering alternative treatment options, like PCSK9 inhibitors, to effectively manage cardiovascular risks in individuals with a high 10-year ASCVD score. Further research is warranted to better understand and mitigate such uncommon side effects associated with statins.
It is important to note that the risk of tendon rupture with statins is relatively low and that its benefits often outweigh the risks. However, if your patient is taking one of these medications and experiences sudden pain or weakness in a tendon, you should consider the possibility of tendon rupture, and we recommend (PCSK9) inhibitors as an alternative choice for these patients.
Declarations
1. Funding: Not applicable
2. Conflicts of interest/Competing interests: The authors declare that there is no conflict of interest to declare.
3. Ethics approval: No additional costs and procedures were imposed on the patient’s family members in this study. We reported the retrograde standard treatment process of the patient. We maintained the patient's privacy.
4. Consent to participate: The patient has consented to participate in this case report.
5. Availability of data and material: The data supporting this study's findings are available from the corresponding author, upon reasonable request.
References
- Salari S, Ghadyani M, Karimi M, Mortezazadeh M, Vahedifard F. Immunohistochemical Expression Pattern of MLH1, MSH2, MSH6, and PMS2 in Tumor Specimen of Iranian Gastric Carcinoma Patients. Journal of Gastrointestinal Cancer. 2022; 53: 192-6.
- Califf RM. Biomarker definitions and their applications. Experimental biology and medicine (Maywood, NJ). 2018; 243: 213-21.
- Rashedi S, Keykhaei M, Pazoki M, Ashraf H, Najafi A, Kafan S, et al. Clinical significance of prognostic nutrition index in hospitalized patients with COVID-19: Results from single-center experience with systematic review and meta-analysis. Nutrition in Clinical Practice. 2021; 36: 970-83.
- Blum A, Simsolo C, Hasin Y. 3-Hydroxy-3-methylglutaryl coenzyme a (HMG-CoA) reductase inhibitors (statins), atherosclerosis and coronary syndromes. Atherosclerosis. 2004; 175: 1-5.
- Hajipour R, Bagheri SM, Ghadamzadeh M, Sadeghi P, Vahedifard F, Salmanipour A. Journal of Organ Transplantation. 2022.
- Taylor BA, Thompson PD. Muscle-related side-effects of statins: from mechanisms to evidence-based solutions. Current opinion in lipidology. 2015; 26: 221-7.
- Sigrid M. Pleiotropic Effects of Statins. In: Sekar Ashok K, editor. Hypercholesterolemia. Rijeka: IntechOpen; 2015; 9.
- Soleimani A, Bavandpour Karvane H, Mortezazadeh M, Mofidi A, Seyyed Mahmoudi ST, Kashani M. Early amiodarone pneumonitis: A case report. Clinical case reports. 2022; 10: e6808.
- Moradi Tabriz H, Obohat M, Vahedifard F, Eftekharjavadi A. Survey of Mast Cell Density in Transitional Cell Carcinoma. Iranian journal of pathology. 2021; 16: 119-27.
- Baik S, Lau J, Huser V, McDonald CJ. Association between tendon ruptures and use of fluoroquinolone, and other oral antibiotics: A 10-year retrospective study of 1 million US senior Medicare beneficiaries. BMJ Open. 2020; 10: e034844.
- Halpern AA, Horowitz BG, Nagel DA. Tendon ruptures associated with corticosteroid therapy. West J Med. 1977; 127: 378-82.
- Campbell M, Kusne S, Renfree KJ, Vikram HR, Smilack JD, Seville MT, et al. Coccidioidal Tenosynovitis of the Hand and Wrist: Report of 9 Cases and Review of the Literature. Clinical Infectious Diseases. 2015; 61: 1514-20.
- Kirchgesner T, Larbi A, Omoumi P, Malghem J, Zamali N, Manelfe J, et al. Drug-induced tendinopathy: From physiology to clinical applications. Joint Bone Spine. 2014; 81: 485-92.
- Eliasson P, Dietrich-Zagonel F, Lundin AC, Aspenberg P, Wolk A, Michaëlsson K. Statin treatment increases the clinical risk of tendinopathy through matrix metalloproteinase release - a cohort study design combined with an experimental study. Sci Rep. 2019; 9: 17958.
- Cano Cevallos EJ, Shaikh DH, Gonzalez J, Sanchez W, Patel M. Tendon rupture associated with concomitant simvastatin and gemfibrozil use: Biological and pharmacokinetic implications. Clin Case Rep. 2019; 7: 1919-22.
- van der Vlist AC, Breda SJ, Oei EHG, Verhaar JAN, de Vos R-J. Clinical risk factors for Achilles tendinopathy: a systematic review. British Journal of Sports Medicine. 2019; 53(21): 1352.
- Deren ME, Klinge SA, Mukand NH, Mukand JA. Tendinopathy and Tendon Rupture Associated with Statins. JBJS Reviews. 2016; 4.
- Ekhart C, de Jong L, Gross-Martirosyan L, van Hunsel F. Muscle rupture associated with statin use. British Journal of Clinical Pharmacology. 2016; 82: 473-7.
- Beri A, Dwamena FC, Dwamena BA. Association Between Statin Therapy and Tendon Rupture: A Case-Control Study. Journal of Cardiovascular Pharmacology. 2009; 53.
- Azar H, Ali A, Mahnaz M, Samira K, Abdolazim V, Morteza G, et al. Effects of Remdesivir on in-Hospital and Late Outcomes of Patients With Confirmed or Clinically Suspected COVID-19: A Propensity Score-Matched Study. Acta Medica Iranica. 2022; 60.
- McFarlane S, Muniyappa R, Francisco R, Sowers J. Pleiotropic Effects of Statins: Lipid Reduction and Beyond. The Journal of clinical endocrinology and metabolism. 2002; 87: 1451-8.
- Basiak M, Kosowski M, Cyrnek M, Bułdak Ł, Maligłówka M, Machnik G, et al. Pleiotropic Effects of PCSK-9 Inhibitors. Int J Mol Sci. 2021; 22.
- Vahedifard F, Mortezazadeh M, Mofidi A, Kashani M, Sharifi Rayeni A. Focal nodular hyperplasia in a 14-year-old child: A case report. Caspian journal of internal medicine. 2021; 12: S460-s3.
- Harirchian MH, Mohammadpour Z, Fatehi F, Firoozeh N, Bitarafan S. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clinical Nutrition. 2019; 38: 2038-44.
- Liao JK, Laufs U. Pleiotropic effects of statins. Annual review of pharmacology and toxicology. 2005; 45: 89-118.
- Shalchi Z, Shirsalimi N, Sedighi I. Aseptic Meningitis, As the First Manifestation of Kawasaki Disease: A Case Report. Journal of Comprehensive Pediatrics. 2020; In Press.
- Mortezazadeh M, Vahedifard F, Ahmadi-Renani S, Salimzadeh A. A woman with a bamboo spine in the thoracic vertebra and normal sacroiliac joint; a 5-years undiagnosed ankylosing spondylitis: Case report and literature review. Rheumatology Research. 2020; 5: 33-8.
- Gallego-Colon E, Daum A, Yosefy C. Statins and PCSK9 inhibitors: A new lipid-lowering therapy. European journal of pharmacology. 2020; 878: 173114.
- Allahyari A, Jernberg T, Hagström E, Leosdottir M, Lundman P, Ueda P. Application of the 2019 ESC/EAS dyslipidaemia guidelines to nationwide data of patients with a recent myocardial infarction: a simulation study. European heart journal. 2020; 41: 3900-9.
- Yang Y, Lu H, Qu J. Tendon pathology in hypercholesterolaemia patients: Epidemiology, pathogenesis and management. Journal of Orthopaedic Translation. 2019; 16: 14-22.
- Mortezazadeh M, Kalantari S, Abolghasemi N, Ranjbar M, Ebrahimi S, Mofidi A, et al. The effect of oral probiotics on CD4 count in patients with HIV infection undergoing treatment with ART who have had an immunological failure. Immunity, Inflammation and Disease. 2023; 11: e913.
- Niyousha Shirsalimi SPNIiROTMIAYOGwSCiPOAJBSR, 10.34297/ AJBSR.2021.13.001922.
- Beri A, Dwamena FC, Dwamena BA. Association between statin therapy and tendon rupture: a case-control study. Journal of cardiovascular pharmacology. 2009; 53: 401-4.
- Kamran R, Mehrzad M, Seyyed Amirhossein E, Seyyed Reza S, Ehsan Kamali y, Nasim Z, et al. Evaluation of overall survival and disease-free survival of adjuvant chemotherapy and hormone therapy in patients with breast cancer. Basic & Clinical Cancer Research. 2023; 14.