Research article
Volume 2, Issue 7
Accuracy of Pulse Oximeter at Different Sensor Locations in Patients with Viral Pneumonia: Comparison of Finger and Earlobe
Farzan Vahedifard1; Zahra Rezaei2; Mehrdad Jafari Tadi3*; Mahsa Fadaei4; Mehdi Kashani5
1Internal Medicine Department, Iran University of Medical Sciences, Tehran, Iran.
2Internal Medicine Department, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3Research Committee, Shahid Beheshti University of Medical Science, Iran.
4Ahvaz Medical School Research Committee, Ahvaz, Iran.
5School of medicine, Tehran university of medical sciences, Tehran, Iran.
Corresponding Author:
Mehrdad Jafari Tadi
Email: mehrdad.j.tadi@gmail.com
Received : Jun 16, 2023 Accepted : Jul 07, 2023 Published : Jul 14, 2023 Archived : www.meddiscoveries.org
Citation: Vahedifard F, Rezaei Z, Tadi MJ, Fadaei M. Accuracy of Pulse Oximeter at Different Sensor Locations in Patients with Viral Pneumonia: Comparison of Finger and Earlobe. Med Discoveries. 2023; 2(7): 1054.
Copyright: © 2023 Tadi MJ. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Since the advent of the COVID-19 pandemic, there has been a significant surge in patients admitted to emergency departments exhibiting hypoxemia yet showing no signs of dyspnea. This intriguing presentation sparked our hypothesis that SpO2 measurements derived from the index finger may not reliably estimate the actual peripheral blood oxygen saturation.
To substantiate our hypothesis, we orchestrated a study that compared the efficacy of SpO2 monitoring at two different sensor locations: the earlobe and the traditionally used index finger. Our objective was to scrutinize the variation in these measurements against the gold-standard reference of arterial blood gas (ABG) analysis. We were particularly interested in understanding whether the measurement site would impact the precision of the SpO2 readings and, consequently, the clinical decision-making process.
Our results revealed that the earlobe-based pulse oximetry readings demonstrated a higher congruence with the SaO2 values derived from the ABG analysis when compared to finger-based measurements, particularly in patients with mild to moderate COVID-19 symptoms. However, for patients exhibiting PaO2/FiO2 ratios less than 250, the index finger measurements were found to be more precise.
Our findings contribute to the evolving understanding of optimal pulse oximetry sensor locations, offering clinicians a more nuanced perspective when interpreting SpO2 readings in the context of viral pneumonia such as COVID-19. This could potentially refine the assessment of patient condition and enhance the effectiveness of clinical decisions in a critical care environment.
Keywords: Happy hypoxia; COVID-19; Silent hypoxia; Pulse oximetry; Micro thrombosis.
Introduction
In recent years, the focus on diagnostic biomarkers as a pivotal tool for diagnosing a spectrum of diseases - ranging from cancer to infectious diseases - has gained significant momentum [1]. The emergence of the COVID-19 pandemic has further highlighted the crucial role of these diagnostic indicators, particularly concerning hypoxia, a condition characterized by low oxygen saturation (SpO2) levels in the body.
An intriguing phenomenon observed since the onset of the COVID-19 pandemic is the presentation of many patients to emergency departments with surprisingly low SpO2 measures, yet without commensurate respiratory distress or cognitive decline [22,23]. This paradoxical condition, wherein patients appear comfortable despite critically low oxygen levels, has been colloquially termed “happy hypoxia” [5-7].
Our study was primarily driven by the hypothesis that SpO2 measurements via the index finger, a conventional site for pulse oximetry, may not accurately reflect the true peripheral blood oxygen saturation in COVID-19 patients. We conjectured that a more central location, specifically the earlobe, might provide a more precise estimation of blood oxygen saturation.
To test our hypothesis, we meticulously designed a study wherein SpO2 monitoring was performed via earlobe and compared against the gold standard - the right index finger-clip pulse oximeter. We further validated these readings with a simultaneous arterial blood gas (ABG) analysis to ensure the reliability of our findings.
Methods
Following the acquisition of informed consent, our study encompassed 27 COVID-19 patients. These participants were admitted to the infectious diseases ward between January and February 2021. The inclusion criteria centered on adult patients diagnosed with COVID-19 pneumonia through SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) testing, and subsequent admittance to the infectious diseases ward.
Several conditions defined our exclusion criteria, including patients’ inability to tolerate 3 minutes of respiration without supplemental oxygen and any drop in SpO2 levels below 80%. Additional exclusion factors involved a body temperature less than 35°C, pre-existing conditions known to affect pulse oximetry accuracy such as sickle cell disease or congenital methemoglobinemia, and patients requiring high inotropic support.
The SpO2 measurements were carried out using a cardiac monitoring device’s pulse oximeter, with the sensor placed on both the finger pad and earlobe. Prior to taking measurements, patients were kept off supplemental oxygen for a 3-minute interval to establish a baseline. Simultaneously, an arterial blood sample was obtained from the femoral artery to enable arterial blood gas (ABG) analysis.
After collecting initial measurements, patients were adequately ventilated using face masks at a rate of 5 L/min. After a minimum of 3 minutes and confirmation of SpO2 stabilization, measurements were again captured from the earlobe and finger. Additionally, a complete blood count (CBC) was collected to ascertain hemoglobin and red blood cell counts, contributing to a comprehensive patient health profile.
We further incorporated a review of patients’ initial CT scores, calculated based on the methodology proposed by Li et al. This involved assigning a score ranging from 0 to 5, according to the extent of viral infiltrates in each lung lobe [8]. By integrating this multidimensional approach, our study aimed to provide a thorough understanding of SpO2 measurement accuracy at different sensor locations in COVID-19 patients.
Results
Our study included 27 patients, whose mean age was 50, with a standard deviation of 11.2. The mean chest CT score, indicating the extent of the lung damage, was 14.6, with a standard deviation of 3.2.
Upon analysis of the SpO2 measurements using the Wilcoxon’s Signed Ranks test, we identified significant differences between the SpO2 values measured at the earlobe and finger. When patients received supplemental oxygen, the mean SpO2 measured at the earlobe was 96.1 (SD: 3.0), significantly higher than the mean SpO2 of 91.4 (SD: 3.0) measured at the finger. The statistical test yielded a Z score of -2.81, with a p-value of 0.005, indicating this difference was statistically significant.
Table 1: Baseline characteristics of study participants.
| Variable | Value |
|---|---|
| Age | 50 ± 11.2 |
| Sex | |
| -male | 24(88.9%) |
| -female | 3(11.1%) |
| Smoking | 6(22.2%) |
| BMI | 28.6 ± 3.4 |
| Pulse Oximeter data | |
| SpO2 without oxygen (%) | |
| -Finger | 85.4 ± 3.0 |
| -Ear | 92.7 ± 4.1 |
| SpO2 with oxygen (%) | |
| -Finger | 91.4 ± 3.0 |
| -Ear | 96.1 ± 3.0 |
| Vital Signs | |
| -SBP (mmHg) | 134 ± 10 |
| -DBP (mmHg) | 87 ± 13 |
| -HR | 91 ± 13 |
| -RR | 27 ± 5 |
| -T (°C) | 37.2 ± 0.6 |
| ABG | |
| -pH | 7.45 ± 0.04 |
| -pCO2 (mmHg) | 31.9 ± 5.3 |
| -HCO3 - (mmol/L) | 21.9 ± 1.9 |
| -pO2 (mmHg) | 55.9 ± 11.6 |
| -SpO2 (%) | 88.5 ± 7.9 |
| Hb (g/dL) | 14.7 ± 1.7 |
| RBC *106 | 5.1 ± 0.7 |
| -PaO2/FiO2 | 279 ± 39 |
| CT score | 14.6 ± 3.2 |
| Hospital Stay (day) | 11 ± 5 |
Moreover, even without supplemental oxygen, the earlobe readings displayed.
Compared to 85.4 (SD: 3.0) at the finger, with a Z score of -2.82 and a p-value of 0.005, further emphasizing the significance of this difference.
As can be inferred from Table 1, the arterial blood gas (ABG) analysis, which serves as the gold standard, generally aligned more closely with the SpO2 values measured via the earlobe than those taken from the finger. This observation was consistent for all but two patients in our study. Notably, these two exceptions had PaO2 /FiO2 values less than 250, indicating severe hypoxemia.
These results suggest that pulse oximetry readings taken from the earlobe are more accurate and reliable for most patients with viral pneumonia, presenting important implications for patient monitoring during the COVID-19 pandemic.
Conclusion and clinical implications
Since the emergence of COVID-19, numerous biomarkers have been studied for diagnosing both acute and chronic stages of the disease [4,9,10]. Previous investigations have highlighted the potential inaccuracies of pulse oximetry readings under conditions of severe and rapid O2 desaturation, hypotension, hypothermia, dyshemoglobinemia, and poor blood perfusion [11,24,25].
In our study involving COVID-19 patients, the earlobe was found to be a more reliable site for pulse oximetry readings than the finger pad. Specifically, average blood oxygen saturation measurements obtained from the earlobe aligned more closely with SaO2 values ascertained by arterial blood gas (ABG) analysis.
This pattern was consistent across our patient sample, with only two exceptions. These two patients, who had PaO2 /FiO2 values less than 250, reflect findings by Clayton and colleagues [13], who found finger sensor readings were more reliable among patients with severe hypoxemia.
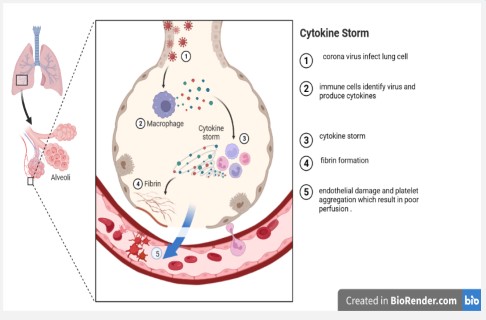
Recent investigations have shed light on the pathology of COVID-19, illuminating how SARS-CoV-2 invasion and subsequent host immune responses can lead to endothelial damage, which can, in turn, induce microvascular thrombosis and microcirculation disorders – conditions found in 91.3% of post-mortem COVID-19 patients [14].
Many clinical trials have confirmed the presence of microcirculatory dysfunction in sepsis, often resulting from NO (nitric oxide) dysregulation, endothelial damage, and functional impairments among various cell types present in the microcirculation [15,16].
In another study focusing on athletes post-training, capillary blood samples taken from the finger pad showed consistently higher lactate concentrations than those from the earlobe taken concurrently [17]. High lactate levels indicate tissue hypoxia, poor perfusion, reduced blood pH, and oxygen debt – conditions conducive to lactate production [6,18].
Our findings, when considered in light of these studies, suggest that the finger pad's microvasculature is more susceptible to acute illnesses than that of the earlobe. This observation holds significance considering recent revelations about the role of COVID-19 in inducing micro-thrombosis and significant perfusion abnormalities, especially in cases of severe viral pneumonia [12,26] (Figure 1).
Further evidence demonstrates that even patients with low Wells scores show signs of perfusion defects in lung segments and subsegments, especially those with persistently high ddimer levels or exertional dyspnea in the post-COVID phase. Moreover, outpatients with mild-to-moderate COVID-19 have an increased thrombotic risk and are often recommended for anticoagulant prophylaxis during their illness [19].
Thus, our research and the cited studies suggest that thrombotic events play a significant role even in mild COVID-19 cases. In contrast to the earlobe, the finger pad appears more susceptible to hypoxic changes, affirming our initial hypothesis and underlining the necessity for further research in this area.
We assume that these extensive thrombi in the capillaries and poor peripheral perfusion may cause the underestimation of the true Spo2 measured through finger pulse oximetry. Compared with the finger pad, the earlobe might benefit from straighter and more central arterial circulation, being less amenable to false SpO2 interpretations through pulse oximeter devices.
Thus, we recommend earlobe pulse oximeter measurements in patients admitted to the emergency room for triage of mild to moderate COVID-19; the greater difference between ear and finger measurement predicts a more serious patient's condition. However, larger studies are needed to confirm our results.
Declarations
Funding: Not applicable
Conflicts of interest/Competing interests: The authors declare no conflicts of interest to declare.
Consent to participate: The patients have consented to participate in this study.
Consent for publication: The participant has consented to the publication of this study.
Availability of data and material: The data supporting this study's findings are available from the corresponding author upon reasonable request.
Code availability: Not applicable.
References
- Salari S, Ghadyani M, Karimi M, Mortezazadeh M, Vahedifard F. Immunohistochemical Expression Pattern of MLH1, MSH2, MSH6, and PMS2 in Tumor Specimen of Iranian Gastric Carcinoma Patients. Journal of Gastrointestinal Cancer. 2022; 53: 192-6.
- Califf RM. Biomarker definitions and their applications. Experimental biology and medicine (Maywood, NJ). 2018; 243: 213-21.
- Rashedi S, Keykhaei M, Pazoki M, Ashraf H, Najafi A, et al. Clinical significance of prognostic nutrition index in hospitalized patients with COVID-19: Results from single-center experience with systematic review and meta-analysis. Nutrition in Clinical Practice. 2021; 36: 970-83.
- Amirkhani M, Ghorbanshiroudi S, Zarbakhsh Bahri M, SeyedAlinaghi S. Mindfulness-Based Compassion-Focused Therapy and a Comparison of Effectiveness of Stress Reduction Program on Self-Compassion of HIV Positive Patients. Journal of Iranian Medical Council. 2021; 4: 311-21.
- Couzin-Frankel J. The mystery of the pandemic’s ‘happy hypoxia’. Science. 2020; 368: 455-6.
- Almodovar MC, Kulik TJ, Charpie JR. Chapter 22 - Assessment of Cardiovascular Function. In: Fuhrman BP, Zimmerman JJ, editors. Pediatric Critical Care (Fourth Edition). Saint Louis: Mosby; 2011; 246-54.
- Hadadi A, Ajam A, Montazeri M, Kafan S, Veisizadeh A, et al. Effects of Remdesivir on in-Hospital and Late Outcomes of Patients With Confirmed or Clinically Suspected COVID-19: A Propensity Score-Matched Study. Acta Medica Iranica. 2022; 60: 407-12.
- Li K, Wu J, Wu F, Guo D, Chen L, et al. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest Radiol. 2020; 55: 327-31.
- Janbazi L, Kazemian A, Mansouri K, Madani SP, Yousefi N, et al. The incidence and characteristics of chronic pain and fatigue after 12 months later admitting with COVID-19; The Post- COVID 19 syndrome. American Journal of Physical Medicine & Rehabilitation.
- Mohammad B, Amir Mohammad A, Amir Ali A, Saina Nezami N, Masoud M, et al. Prevalence of olfactory dysfunction in COVID-19 patients. Journal of Craniomaxillofacial Research. 2021; 8.
- Seifi S, Khatony A, Moradi G, Abdi A, Najafi F. Accuracy of pulse oximetry in detection of oxygen saturation in patients admitted to the intensive care unit of heart surgery: comparison of finger, toe, forehead and earlobe probes. BMC Nurs. 2018; 17: 15.
- Vahedifard F, Chakravarthy K. Nanomedicine for COVID-19: the role of nanotechnology in the treatment and diagnosis of COVID-19. Emergent Materials. 2021; 4: 75-99.
- Pazoki M PP, Montazeri M, Kafan S, Sheikhvatan M, Hadadi A. Risk Stratification for In-hospital Mortality in Adult Patients with COVID-19. J Iran Med Counc. 2021; 4: 322-34.
- Clayton DG, Webb RK, Ralston AC, Duthie D, Runciman WB. Pulse oximeter probes. A comparison between finger, nose, ear and forehead probes under conditions of poor perfusion. Anaesthesia. 1991; 46: 260-5.
- Chen W, Pan JY. Anatomical and Pathological Observation and Analysis of SARS and COVID-19: Microthrombosis Is the Main Cause of Death. Biological Procedures Online. 2021; 23: 4.
- Miranda M, Balarini M, Caixeta D, Bouskela E. Microcirculatory dysfunction in sepsis: pathophysiology, clinical monitoring, and potential therapies. American Journal of Physiology-Heart and Circulatory Physiology. 2016; 311: H24-H35.
- Mortezazadeh M, Kalantari S, Abolghasemi N, Ranjbar M, Ebrahimi S, et al. The effect of oral probiotics on CD4 count in patients with HIV infection undergoing treatment with ART who have had an immunological failure: Immun Inflamm Dis. 2023; 11: e913.
- Feliu J, Ventura JL, Segura R, Rodas G, Riera J, et al. Differences between lactate concentration of samples from ear lobe and the finger tip. J Physiol Biochem. 1999; 55: 333-9.
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, et al. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-40.
- Ozturk BC, Atahan E, Gencer A, Harbiyeli DO, Karabul E, et al. Investigation of perfusion defects by Q-SPECT/CT in patients with mild-to-moderate course of COVID-19 and low clinical probability for pulmonary embolism. Ann Nucl Med. 2021; 35: 1117-25.
- Harirchian MH, Mohammadpour Z, Fatehi F, Firoozeh N, Bitarafan S. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clinical Nutrition. 2019; 38(5): 2038-44.
- Janbazi Lobaneh, Kazemian Alireza, Mansouri Kourosh, Madani Seyed Pezhman, Yousefi Naseh, Vahedifard Farzan, Raissi Gholamreza. The incidence and characteristics of chronic pain and fatigue after 12 months later admitting with COVID-19; The PostCOVID 19 syndrome. American Journal of Physical Medicine & Rehabilitation. 2022 :10.1097/PHM.0000000000002030.
- Rezaei M, Molani S, Firoozeh N, Abbasi H, Vahedifard F, Orouskhani M. Evolving Tsukamoto Neuro Fuzzy Model for Multiclass Covid 19 Classification with Chest X Ray Images. arXiv preprint arXiv:230510421. 2023.
- Soleimani, A., Mortezazadeh, M., Mofidi, A., ST, S. M., & Kashani, M. Early amiodarone pneumonitis: A case report. Clinical Case Reports. 2022; 10(12): e6808-e6808.
- Mortezazadeh M, Kalantari S, Abolghasemi N, Ranjbar M, Ebrahimi S, Mofidi A, et al. The effect of oral probiotics on CD4 count in patients with HIV infection undergoing treatment with ART who have had an immunological failure. Immunity, Inflammation and Disease. 2023: 11(6).
- Roudini K, Mirzania M, Emami SA, Safayi SR, Kamali yazdi E, Zarifi N, Mortezazadeh M, Rafiee S, Mofidi A, Kashani M, Mansouri E sadat, Rasekhi Siahkal mahalleh M, Khajeh Mehrizi A. Evaluation of overall survival and disease-free survival of adjuvant chemotherapy and hormone therapy in patients with breast cancer. Basic Clin Cancer Res. 2023; 14(2): 87-94.