Research Article
Volume 2, Issue 7
Protective Effects of Silymarin against Chronic Exposure to Manganese-Induced Hematological Alterations, Biochemical Perturbations and Genotoxicity in Rats
Khadija Boukholda1; Yassine Chtourou1; Fatma Makni-Ayadi2; Choumous Kallel3; Fatiha Chigr4; Hamadi Fetoui1*
1Laboratory of Toxicology-Microbiology and Environmental Health (17ES06), Faculty of Sciences of Sfax, University of Sfax, BP1171, 3000 Sfax, Tunisia.
2Laboratory of Biochemistry, CHU Habib Bourguiba, University of Sfax, 3029 Sfax, Tunisia.
3Laboratory of Hematology, CHU Habib Bourguiba, University of Sfax, 3029 Sfax, Tunisia.
4Biological Engineering Laboratory, Faculty of Sciences and Techniques, Sultan Moulay Slimane University, Beni Mellal, Morocco.
Corresponding Author :
Hamadi Fetoui
Tel: +216-50680837, Fax: +216-74-274-437
Email: fetoui_hamadi@yahoo.fr/hamadi.fetoui@fss.usf.tn
Received : Jun 11, 2023 Accepted : Jul 03, 2023 Published : Jul 10, 2023 Archived : www.meddiscoveries.org
Citation: Boukholda K, Chtourou Y, Makni-Ayadi F, Kallel C, Chigr F, et al. Protective Effects of Silymarin against Chronic Exposure to Manganese-Induced Hematological Alterations, Biochemical Perturbations and Genotoxicity in Rats. Med Discoveries. 2023; 2(7): 1052.
Copyright: © 2023 Fetoui H. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Manganese (Mn), a trace metal, is essential for maintaining the normal regulation of many biochemical and cellular processes. However, accumulation of Mn due to excessive environmental exposure leads to neurological impairment, biochemical lesions, and genotoxic effects which results in a sequalae of physiologic and pathologic responses. Natural compounds such as silymarin, a bioactive flavonoid present in Milk Thistle (Silybum marianum L.), might be beneficial for the treatment of those disorders. Therefore, this study aimed to investigate whether and how silymarin protects against manganese-induced toxicity in aged rats. Animals were divided into four groups. The first group was used as control. Group 2 was orally treated with Manganese (Mn, 50 mg/kg) for 16-weeks. Group 3 was orally treated with silymarin (100 mg/kg) for 16-weeks. Groups 4 was co-treated with SIL and Mn (Mn+SIL). Various biochemical related to liver and kidneys functions, hematological and genotoxic biomarkers were assessed. The results indicate that Mn-intoxicated rats for 16-weeks display significantly higher levels of plasma markers related to kidneys and liver functions (AST, ALT, PAL γGT, urea, uric acid and CK-MB) than normal control animals. Moreover Haematological parameters (RBC, Hb, Hct and PLT) were significantly decreased in the Mn group compared to controls with a significant difference in erythrocyte osmotic fragility (p≤0.05). Moreover, a genotoxic effect was observed in rat peripheral blood after 16-weeks of exposure to Mn evidenced by a significant increase in the frequency of micronucleus (MN). Co-administration of SIL (100 mg/kg) resulted in a significant reversal of hematological and biochemical markers in Mnintoxicated rats.
In conclusion, SIL exhibits positive effects on some hematological characteristics and osmotic fragility in erythrocytes and improves biochemical parameters related to hepatic and kidenys functions in case of chronic manganese toxicity.
Keywords: Silymarin; Manganese; Chronic toxicity; Hepato-renal toxicity; Micronucleus.
Introduction
Contamination with heavy metals is a serious ecological problem for humans and animals. Although metals are biologically important, they are usually required in trace amounts, excessive metal accumulation in various organs induces various detrimental intracellular events (oxidative stress, mitochondrial dysfunction, DNA fragmentation, protein misfolding, endoplasmic reticulum (ER) stress, autophagy dysregulation, and the activation of apoptosis) [1]. The ubiquity of these pollutants in our daily lives is the cause of a quarter to a third of the diseases that occur in developing countries and is the direct cause of 2 to 5% of mortality [2]. Some metals such as cadmium (Cd), lead (Pb), mercury (Hg), or aluminum (Al) have no biological function and only have toxic effects. Other metals such as copper (Cu), manganese (Mn), iron (Fe), and zinc (Zn) are essential for many biological reactions such as the synthesis of DNA, RNA, and metalloproteins as catalysts and coenzymes. However, overexposure to environmental metals via industrial occupation or contaminated drinking water can lead to toxic effects in the brain, the liver, and the cardiovascular system through differents pathways such as the production of free radicals and the generation of oxidative stress [3].
Although Mn is an essential element for the human body which plays an important role in a number of physiological processes by serving as a constituent of some enzymes and an activator of others involved in the regulation of amino acid, protein, lipid, and carbohydrate metabolism [4]. Excessive manganese exposure is well reported to be associated with several cellular dysfunctions [5]. Mn is generally believed to exert cellular toxicity via a number of mechanisms, including the induction of free radical production; direct or indirect formation of reactive oxygen species (ROS) [6,7]; changes in the functions of all neurotransmission pathways [8,9]; and disruption of the homeostasis of Ca, Fe, and trace minerals [10,11]. Epidemiological evidence indicates that co-exposure to multiple heavy metals such as Mn is associated with hematological and biomedical changes [12,13]. Experimental studies in rats have also reported that increased oxidative damage may mediate the association of co-exposure to heavy metals with these adverse health effects [14,15]. Different approaches may apply to minimize the severity of Mn toxicity. Considering the relationship of Mn exposure with cellular toxicity and its role in elemental homeostasis, the administration of antioxidants biomolecules may be protective in Mn toxicity [16].
Silymarin is one of the most promising natural products, extracted from the fruits and seeds of milk thistle (Silybum marianum) [17]. SIL is regarded as the most effective drug for treating nearly all types of liver diseases, specifically alcoholic liver disease, chronic and acute viral hepatitis, and toxin-mediated liver impairments [18]. In addition, it is implicated in the treatment of various diseases of different organs including the kidneys, prostate, lungs, and nervous system [19]. In addition to hepatoprotection, SIL has been reported to prevent different kinds of cancers such as lung, bladder, prostate, breast, and ovarian cancers [20]. Recently, SIL gained prominence as a neuroprotective compound as it has an antioxidant activity by scavenging free radicals that inhibit lipid peroxidation and anti-inflammatory impacts in the CNS and can penetrate the CNS via the blood–brain barrier (BBB) [21]. Besides, it has anti-inflammatory action as well as hepatoprotective properties through enhancing superoxide dismutase activity and glutathione activity coupled with high antitumor-promoting activity [22].
In this study, in order to make a comprehensive assessment of increased manganese doses on aged rats and to improve our understanding of its non-safety effects, hematological, biochemical, and molecular analysis were determined. To our knowledge, the current work is the first to investigate the longterm treatment of Mn to rats, as well as the protective impact of silymarin against chronic manganese exposure.
Materials and methods
Animals and experimental design
Fourty adult male Wistar rats weighing about 250 ± 10 g were purchased from the Central Pharmacy (SIPHAT, Tunisia). Animals were housed under standard (22 ± 2◦C, humidity: 60 ± 5%) laboratory conditions, maintained on a 12 h light/dark cycle with free access to food and water [23]. The doses of manganese and silymarin in the experiments was selected based on our previous studies [24]. The period of treatment was 16-weeks. The rats were randomly divided into four groups of ten animals each (n=10). Animals of the first group received only physiological saline (NaCl 0.9%) and served as the control group. The second group received orally a dose of 50 mg/kg bw of manganese (Mn) for 16-weeks. The third group (Mn+SIL) rats received manganese at dose of 50 mg/Kgbw and silymarin at dose 100 mg/kgbw. In the fourth group (SIL) rats received only silymarin at dose od (100 mg/kgbw). The oral route was chosen to avoid gastrointestinal tract effects, to avoid any loss of products during administration, and because it provides high bioavailability of Mn compared to other routes of administration [27]. After the period of treatment, animals in different groups were sacrificed by cervical decapitation to avoid stress conditions, and blood samples were collected in EDTA tubes for hematological parameters analysis. Others samples were collected, centrifuged and stored at -80°C for biochemical analysis. All animal procedures were conducted in strict conformation with the local Institute Ethical Committee Guidelines for the Care and Use of Laboratory Animals of our faculty.
Hematological analysis
Blood samples were obtained from each rat and collected in separate test tubes with, EDTA (ethylene diamine tetra-acetic acid). Then, Blood samples were immediately processed for hematological parameters using Automated Hematological Analyzer (SYSMEX RX 21, Japan). The parameters measured are red and white blood cells, hemoglobin (Hb), hematocrit (Ht), mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular hemoglobin content (MCHC).
Osmotic fragility and hemolysis curve
The measurement of the osmotic fragility of blood and the analysis of the hemolysis curve were performed according to the previously described methodology [28]. Briefly, to each of a series of test tubes containing 1 ml of NaCl solution in the concentration range (0–9) g/l of NaCl buffered in 10 mM PBS, pH 7.4, 10 µl of blood was added, mixed, and incubated for 30 min. After 1 hour of incubation at room temperature, the erythrocyte suspension was centrifuged at 1000 g for 10 minutes. The level of free hemoglobin in the obtained supernatant was determined spectrophotometrically by measuring the absorbance at 540 nm against distilled water. The hemolysis curve was performed twice, the first time initially estimating the osmotic fragility value and the second time with high precision and resolution, especially in the range of NaCl concentrations, where a sharp increase in absorbance was observed. The value of osmotic fragility is the concentration of NaCl at which half of the blood cells were hemolyzed. The NaCl concentration values to achieve 50% hemolysis (H 50) for each sample were calculated from the percent hemolysis values obtained at different NaCl concentrations using GraphPad Prism software.
Determination of serum biochemical parameters
The biochemical parameters performed in this study are general workup that allows the exploration of the main hepatic and renal functions. Enzyme activities are determined by colorimetric assay using commercial kits from Biomaghreb.
The parameters assessing liver function measured in this study were aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT). Clinical biochemistry uses nitrogen excretion tests to demonstrate renal failure. We will focus here on urea and creatinine excretion and the determination of creatine kinase (CK) reactivated by N-acetylcysteine to explore muscle damage.
Micronucleus assay (MNA)
The MNA is an important in vivo and in vitro biomarker, extensively used in molecular epidemiology and cytogenetic damage in populations exposed to genotoxic agents [29]. The blood sample is deposited at the end of a degreased histological slide, then spread with a slide. The smears are immediately dried in fresh air. They are then fixed with methanol for 5 min, stained with Acridine orange (100 µg/ml) or Giemsa for 3 min, then washed twice with phosphate buffer (pH 6.8). The reading of the blood smears is performed with a fluorescence microscope (Olympus Model BX 51) for the slides stained with acridine orange. Counts were performed on 4 slides per rat with an objective of × 1000. Cell counts were determined using Photoshop® CS5 software (Adobe Systems). Ten thousand erythrocytes were counted per group.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 9 for Microsoft (GraphPad Software, San Diego, CA). Parameters were expressed as mean ± standard deviation (mean ± SD). Multiple comparisons were performed using one-way ANOVA followed by Tukey’s post hoc test.
Results
Effects of silymarin against chronic administration of manganese on hematological parameters
All the hematological parameters measured for the different tested groups are presented in Table 1. Our results show that only white blood cells, hematocrit, and platelets are altered after treatment with manganese compared to control rats. We observe a statistically significant decrease in hematocrit and an increase in white blood cells and platelets. While in the manganese-contaminated and silymarin-treated batches, there was a significant partial restoration compared to the manganesetreated group without reaching their normal levels except for hematocrit. Besides, Silymarin did not produce significant change on any of the hematological parameters tested compared to the control.
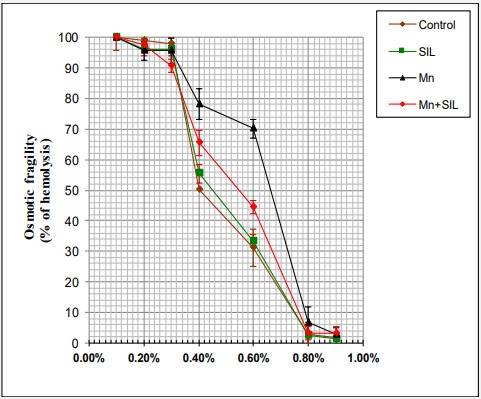
Effects of silymarin against chronic administration of manganese on erythrocyte osmotic fragility
The results of the osmotic fragility study of red blood cells from control and manganese and/or silymarin-exposed rats were shown in Figure 1. The study of the osmotic fragility curves of the different groups of red blood cells showed that those of the red blood cells of the manganese-exposed rats were deviated to the right compared to those of the red blood cells of the control rats. Similarly, there was a significant increase in the percentage of hemolysis in the Mn group in comparison with the control group at 50% hemolysis. The NaCl concentration at 50% hemolysis in control was 0,4 ± 0,04 while 50% hemolysis in the Mn group was 0,64 ± 0,05 as shown in Figure 1. In contrast, no significant improvement was observed between the control and Mn + SIL group (Table 2).
Effects of silymarin against chronic administration of manganese on plasmatic biomarkers of renal function
Table 3 shows some plasmatic biomarkers of renal function in control and manganese-treated rats and manganese plus silymarin combinations after 16-weeks of treatment.
Our results show a significant increase in serum uric acid levels in the manganese-treated group compared to the control group. While there was no significant variation in plasma urea and creatinine levels. The results also showed a recovery by a significant decrease in the groups receiving the Mn+ SIL compared to the Mn group. The co-administration of silymarin inhibited the increase in uric acid levels in Mn-treated rats.
Effects of silymarin against chronic administration of manganese on plasmatic biomarkers of liver function
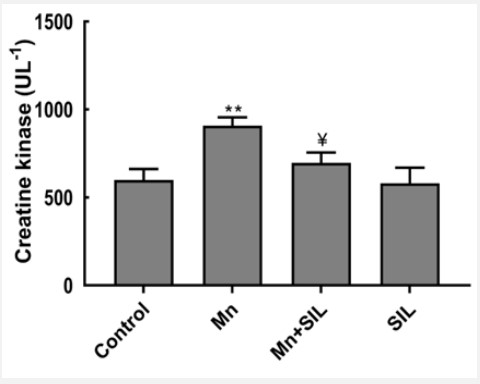
Table 4 summarizes selected plasma biomarkers of liver and muscle function of control and manganese and manganeseplus-silymarin-treated rats after 16-weeks of treatment. The results obtained showed changes resulting from the release of liver injury markers (ASAT and GGT). Alkaline phosphatase, ALT and GGT increased significantly in Mn group (p<0.01, p<0.001 respectively) in comparison with the control group while ALT and GGT decreased significantly (p<0.01, p<0.001 respectively) in Mn+SIL group in comparison with Mn group. However, no significant difference was seen in AST in comparison with control. The co-administration of silymarin partially attenuated the Mninduced hepatotoxicity as shown by the decrease in these enzyme activities. According to our results, lipid parameters were disturbed by manganese, indeed, we recorded the increase in serum triglyceride concentrations in manganese-contaminated rats compared to controls. Besides, we notice a recovery by a significant decrease of triglycerides in rats treated with Mn+SIL compared to rats treated with Mn (Table 4). Concerning the plasma biomarkers of muscle function, the results of the creatine kinase activity assay show a highly significant increase in its activity (p<0.001) in the manganese-exposed batch compared to the control. While in the group treated with manganese and co-administered with silymarin (Mn+SIL), a significant decrease (p<0.01) was recorded compared to the manganesetreated group (Figure 2).
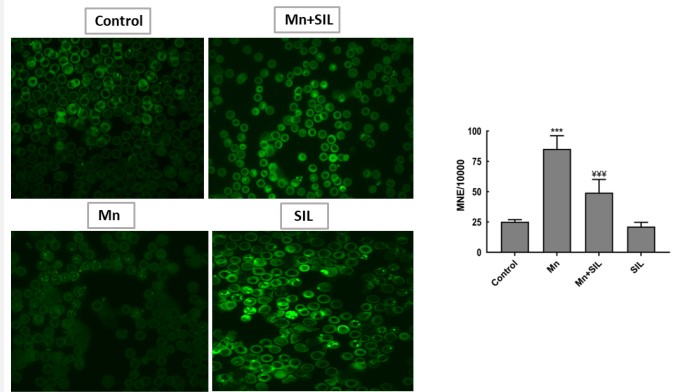
Genotoxicity: Micronucleus assay
Regarding the Micronucleus assay, after chronic exposure, an increase of micronuclei in erythrocytes of rats exposed to 50 mg /kg of Mn was observed in relation to the control animals. In contrast, it was observed a decrease of micronuclei in the erythrocytes of rats exposed to both concentrations (50 mg/kg Mn and 100 mg/kg SIL) when compared to the control group. These results suggest the genotoxic effect of manganese when exposed. However, Silymarin decreases the severity of the lesions produced by manganese (Figure 3).
Discussion
Manganese (Mn), an essential trace metal for the health of organisms, is widely used in industry and agriculture, such as welding and in pesticides [30]. However, humans can be exposed to Mn through water, food, air, or contact with industrial products. Mn is present in all body tissues. The highest levels of Mn are located in the liver, kidney, pancreas, and adrenal glands and the intermediate concentrations are detected in the brain, heart, and lungs [31]. Indeed, with extensive use of Mn in industry and agriculture, Mn concentration in the water environment becomes high, and it has become a kind of water environment pollutant. Consequently, excess Mn in the water environment causes adverse biological alterations in aquatic organisms [30] and animal tissues [32]. However, insufficient literature is reported regarding the exact mechanisms of chronic toxicity (16-weeks) of Mn in aged rats and the therapeutic usage of the drug to counter this side effects. The present study aimed to investigate the potential role of Silymarin on the Hematological parameters and serum markers related to the renal and hepatic functions in aged rats exposed to Mn.
Table 1: Hematological parameters of silymarin (SIL) and nonsilymarin treated animals after 16-weeks of treatment to manganese.
| Parameters | Control | SIL | Mn | Mn+SIL |
|---|---|---|---|---|
| RBC (1012/L) | 8.35 ± 0.45 | 8.76 ± 0.84 | 8.98 ± 0.39 | 8.12 ± 0.58 |
| WBC (109/L) | 8.65 ± 1.80 | 9.50 ±1.45 | 15.13 ± 0.33*** | 12.23 ± 0.90* |
| HGB (g/L) | 143.30 ± 3.86 | 144.5 ± 5.19 | 135.0 ± 5.35 | 136.8 ± 8.30 |
| HCT (%) | 45.37 ± 0.80 | 47.33 ± 1.44 | 39.47 ± 0.55*** | 44.50 ± 2.00* |
| VGM (fl) | 52.20 ± 0.92 | 51.95 ± 1.67 | 50.88 ± 1.48 | 51.45 ± 1.20 |
| TCMH (pg) | 16.57 ± 0.63 | 16.17 ± 0.77 | 16.00 ± 0.80 | 16.85 ± 0.46 |
| CCMH (g/dl) | 31.70 ± 0.90 | 31.08 ± 1.24 | 32.48 ± 0.67 | 31.73 ± 0.69 |
| PLT (109/L) | 803.7 ± 32.87 | 792.0 ± 67.12 | 1087 ± 41.57*** | 950.0 ± 44.51* |
RBC: Red Blood Cells; WBC: White Blood Cells; HGB: Hemoglobin; HCT: Hematocrit; MCHC: Mean Corpuscular Hemoglobin; TCMHC: Mean Corpuscular Hemoglobin Concentration; PLT: Platelets; VGM: Mean Red Blood Cell.The results are presented as mean values ± standard deviation. The number of an29imals n=10. ** p<0.01; *** p<0.001 comparison with control group. ¥ p<0.05 comparison with Mn group.
Table 2: Effects of manganese and/or silymarin exposure on osmotic fragility of erythrocytes (H50).
| Groups | Control | SIL | Mn | Mn+SIL |
|---|---|---|---|---|
| 50% of Hemolysis | 0.4 ± 0.04 | 0.45 ± 0.03 | 0.64 ± 0.05*** | 0.49 ± 0.05** |
The results are presented as mean values ± standard deviation. The number of animals n=10. ** p<0.01; *** p<0.001 comparison with control group. ¥ p<0.05 comparison with Mn group.
Table 3: Changes in serum urea, creatinine, and uric acid concentration after 12 months in control (T), silymarin-treated (SIL), manganese-exposed (Mn), and manganese-treated and silymarinco-administered (Mn+SIL) rats.
| Parameters | Control | SIL | Mn | Mn+SIL |
|---|---|---|---|---|
| Urea (g/L) | 0.30 ± 0.04 | 0.29 ± 0.10 | 0.33 ± 0.09 | 0.31 ± 0.05 |
| Creatinine (mg/L) | 9.13 ± 0.66 | 9.65 ± 1.49 | 7.58 ± 1.86 | 9.48 ± 0.34 |
| Uric Acid (mg/L) | 11.82 ± 2.06 | 12.50 ± 2.36 | 24.07 ± 3.27** | 15.43 ± 3.18* |
The results are presented as mean values ± standard deviation. The number of animals n=10. ** p<0.01; *** p<0.001 comparison with control group. ¥ p<0.05 comparison with Mn group.
Table 4: Variation in enzymatic activity of aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), Gamma Glutamyl transferase (GGT), creatine kinase (CK), alkaline phosphatase (PAL) after 16-weeks months of control (T), silymarin-treated (SIL), manganese-exposed (Mn) and manganese-treated and silymarin co-administered (Mn+SIL) rats.
| Parameters | Control | SIL | Mn | Mn+SIL |
|---|---|---|---|---|
| ASAT (U/L) | 68.58 ± 10.52 | 72.75 ± 8.363 | 77.33 ± 9.26 | 69.42 ± 11.65 |
| ALAT (U/L) | 39.92 ± 1.90 | 39.67 ± 6.14 | 72.92 ± 9.951** | 53.42 ± 3.02* |
| GGT | 9.64 ± 2.18 | 9.88 ± 0.71 | 18.36 ± 1.20*** | 13.44 ± 1.148** |
| PAL (U/L) | 56.92 ± 9.18 | 59.58 ± 4.42 | 60.13 ± 4.17 | 49.50 ± 7.27 |
| Glucose (g/L) | 0.83 ± 0 .04 | 0.87 ± 0.08 | 0.89 ± 0.04 | 0.82 ± 0.05 |
| Cholesterol (g/L) | 0.68 ± 0.14 | 0.55 ± 0.15 | 0.52 ± 0.058 | 0.64 ± 0.17 |
| Triglycerides (g/L) | 1.565 ± 0.34 | 1.64 ± 0.15 | 2.97 ± 0.83** | 1.75 ± 0.18** |
Hematological parameters show conspicuous and significant changes in response to any kind of toxic stress. Blood is a sensitive index of the physiological changes in animals to any environmental contaminant. Herein, alteration of the hematological parameters and the immune system after chronic exposure to Mn administration has been evidenced. Our results show that white blood cells, hematocrit, and platelets are altered after treatment with manganese compared with control rats. We observe a statistically significant decrease in hematocrit and an increase in white blood cells and platelets. While in the manganese-contaminated and silymarin-treated group, there was a significant partial restoration compared to the manganesetreated group without reaching their normal levels except for hematocrit. Similar results were also reported by [33] following sub-chronic intraperitoneal exposure to manganese in the order of 6 mg Mn/kg body weight. In contrast, previous studies [34] showed that silymarin resulted in an improvement in these parameters following chronic lead exposure. This alteration may indicate the toxic effect of Mn on hemoglobin synthesis during the maturation of red blood cells during their formation in the bone marrow, or an anemic state due to hypoxia, iron, cobalamin or folic acid. Furthermore, the decrease in red blood cells and hemoglobin may be related to the inhibition of the circulating erythropoietin hormone [35].
The hemolysis suggested by the increase of osmotic fragility (H50) in the treated group (Mn) could be explained by the denaturation of cell membrane proteins by electrostatic interactions and the generation of reactive oxygen species (ROS). In fact, Free radicals generated by hemoglobin in the erythrocyte, which is a major source producing radicals on interaction with xenobiotics may have also led to increased erythrocyte membrane fragility and hemolysis.
Serum enzymes, including γGT, ALT, ASAT, and PAL have often been used in the evaluation of hepatic disorders. An increase in the activities of these enzymes is indicative of active liver damage or decreased liver uptake, and conjugation after Mn exposure. The results suggest the negative impact of Mn on biochemical parameters of aged rats. This effect could be explained by hepatic lysis and a decrease in synthetic liver activity. Moreover, the co-administration of silymarin showed an improvement of biochemical parameters in the elimination of Mn toxicity.
Kidney indices of toxicity (urea, uric acid, and creatinine) in our experimental groups were investigated. An increase in the levels of these serum markers was found in the rats intoxicated with Mn. Our results are in accordance with several studies showing that uric acid is a powerful scavenger of free radicals and provides 60% of free-radical scavenging capacity in plasma [36]. The rise of such compounds is considered a clear sign of renal dysfunction, in which they should be filtered and poured out by the kidney. Furthermore, a previous study [20] also found that an increase in blood urea was closely associated with histological alterations in the kidney that were degenerative and disrupted the transport system of biochemical elements.
The increase in triglyceride concentrations in the Mn treated group is probably the result of apoptosis and a possible peroxidation of membrane lipids. On the other hand, we notice a recovery by a significant decrease in triglycerides in rats treated with Mn+SIL compared to rats treated with Mn. [37] results showed that regular intake of silymarin lowered triglyceride levels in rats fed a high-fat diet for twelve weeks. Silymarin appears to offer good liver protection and antioxidant potential against hepatocellular damage [37].
Creatine kinase (CK) is a mitochondrial enzyme that catalyzes the conversion of creatine to phosphocreatine, coupled with the conversion of adenosine triphosphate (ATP) to adenosine diphosphate (ADP). The results of the creatine kinase activity assay show a highly significant increase (p<0.001) in the manganese-exposed group compared to the control. While in the group treated with Mn+SIL, a significant decrease (p<0.01) was recorded compared to the manganese-treated group.
Among the effects induced by manganese regularly cited are genotoxic effects. The ability of Mn to interact with nucleotides (DNA, RNA, and ribosome) has been demonstrated in vitro [39]. Our results showed an increase in the frequency of micronuclei in the peripheral blood of rats after chronic exposure to manganese. These results suggest the genotoxic effect of this heavy metal. Although no studies have been done on rats, some metals, such as arsenic and aluminum, have no biological functions and only exert toxic actions by increasing the frequency of micronuclei in the peripheral blood [40]. Silymarin decreases the severity of the lesions produced by manganese. [41] demonstrated that silymarin decreased the number of micronuclei in mice induced by ribavirin [41].
Conclusion
In conclusion, these findings indicate that Mn has a deleterious impact on hematological, biochemical parameters, and genotoxicity in male rats after a long-term exposure and silymarin may be a potential natural compound to attenuated these deleterious effects.
Declarations
Acknowledgements: This work was financially supported by the Tunisian Ministry of higher education and scientific research and in part supported by the PRD program for Research and innovation between Tunisia and Morocco “project number 20/PRD-10”.
Consent for publication: Not applicable.
Availability of data and materials: All data is provided in the manuscript and in additional files.
Competing interests: The authors declare that they have no competing interests.
Authors’ contributions: KB, YC, CK, FMA, FC and HF participated in the research design. The experiments were performed by KB and YC. Data were analyzed by CK, FMA and HF. FC and HF contributed to the writing of the manuscript. All authors have read and approved the final version of the manuscript.
Conflict of interest: The authors declare that they have no conflict of interest.
References
- Wu G, Xiao X, Feng P, Xie F, Yu Z, et al. Gut remediation: A potential approach to reducing chromium accumulation using Lactobacillus plantarum TW1-1. Sci. Rep. 2017; 7: 15000.
- Jiang X, Gu S, Liu D, Zhao L, Xia S, et al. Lactobacillus brevis 23017 Relieves Mercury Toxicity in the Colon by Modulation of Oxidative Stress and Inflammation Through the Interplay of MAPK and NF-κB Signaling Cascades. Front. Microbiol. 2018; 9: 2425.
- Mocchegiani E, Costarelli L, Giacconi R, Piacenza F, Basso A, et al. Micronutrient (Zn, Cu, Fe) -gene interactions in ageing and inflammatory age-related diseases: implications for treatments. Ageing Res Rev. 2012; 11: 297-319.
- Jiang WD, Wu P, Tang RJ, Liu Y, Kuang SY, et al. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Research International. 2016; 89: 670-678.
- Kitada M, Xu J, Ogura Y, Monno I, Koya D. Manganese superoxide dismutase dysfunction and the pathogenesis of kidney disease. Frontiers in physiology. 2020; 11: 755.
- Zhu S, Ho SH, Jin C, Duan X, Wang S. Nanostructured manganese oxides: natural/artificial formation and their induced catalysis for wastewater remediation. Environmental Science: Nano. 2020; 7: 368-396.
- Wang P, Liang C, Zhu J, Yang N, Jiao A, et al. Manganese-based nanoplatform as metal ion-enhanced ROS generator for combined chemodynamic/photodynamic therapy. ACS applied materials & interfaces. 2019; 11: 41140-41147.
- Tinkov AA, Paoliello MM, Mazilina AN, Skalny AV, Martins AC, et al.. Molecular targets of manganese-induced neurotoxicity: a five-year update. International journal of molecular sciences. 2021; 22: 4646.
- Soares ATG, de Castro Silva A, Tinkov AA, Khan H, Santamaría A, et al. The impact of manganese on neurotransmitter systems. Journal of Trace Elements in Medicine and Biology. 2020; 61: 126554.
- Nakata T, Creasey EA, Kadoki M, Lin H, Selig MK, et al. A missense variant in SLC39A8 confers risk for Crohn’s disease by disrupting manganese homeostasis and intestinal barrier integrity. Proceedings of the National Academy of Sciences. 2020; 117: 28930- 28938.
- Fujishiro H, amp; Kambe T. Manganese transport in mammals by zinc transporter family proteins, ZNT and ZIP. Journal of Pharmacological Sciences. 2022; 148:125-133.
- D’Costa AH, Shyama SK, MK PK, Verenkar VS, Tangsali RB. Genotoxic effect of manganese and nickel doped zinc ferrite (Mn 0.3 Ni 0.3 Zn 0.4 Fe 2 O 4) nanoparticle in Swiss albino mouse Mus musculus. Indian Journal of Experimental Biology (IJEB). 2020; 59: 25-32.
- Akhtar K, Javed Y, Muhammad F, Akhtar B, Shad NA, et al. Biotransformation and toxicity evaluation of functionalized manganese doped iron oxide nanoparticles. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2021; 109: 1563-1577.
- Xu J, Zhao M, Liu X, & Xu Q. Effects of heavy metal mixture exposure on hematological and biomedical parameters mediated by oxidative stress. In ISEE Conference Abstracts. 2020.
- Paithankar JG, Saini S, Dwivedi S, Sharma A, &, Chowdhuri DK. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere. 2021; 262: 128350.
- Fu Z, &, Xi S. The effects of heavy metals on human metabolism. Toxicology mechanisms and methods. 2020; 30: 167-176.
- Bijak M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.) Chemistry, bioavailability, and metabolism. Molecules. 2017; 22: 1942.
- Pal PK, A Samii et DB. Calne. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999; 20: no. 2-3: 227–38.
- Park NH, JK Park Y, Choi CI. Yoo CR, Lee, H. et al. Whole blood manganese correlates with high signal intensities on T1-weighted MRI in patients with liver cirrhosis. Neurotoxicology. 2003; 24: 6: 909–15.
- Gazak R, Walterova D, &, Kren V. Silybin and silymarinnew and emerging applications in medicine. Current medicinal chemistry. 2007; 14: 315-338.
- Gillessen A, Schmidt HHJ. Silymarin as supportive treatment in liver diseases: A narrative review. Advances in therapy. 2020; 37: 1279-1301.
- Singh M, Kadhim MM, Turki Jalil A, Oudah SK, Aminov Z, et al. A systematic review of the protective effects of silymarin/silibinin against doxorubicin-induced cardiotoxicity. Cancer Cell International. 2023; 23: 88.
- Boukholda K, Gargouri B, Aouey B, Attaai A, Abd Elkodous M, et al. Subacute silica nanoparticle exposure induced oxidative stress and inflammation in rat hippocampus combined with disruption of cholinergic system and behavioral functions. NanoImpact. 2021; 24: 100358.
- Vezer T, Kurunczi A, Naray M, Papp A, Nagymajtenyi L. Behavioral effects of subchronic inorganic manganese exposure in rats. Am. J. Ind. Med. 2007; 50: 841– 852.
- Tovmasyan A, Sheng H, Weitner T, Arulpragasam A, Lu M, et al. Design, mechanism of action, bioavailability and therapeutic ffects of mn porphyrin-based redox modulators. Medical Principles and Practice. 2013; 22: 103-130.
- Walski T, Grzeszczuk-Kuć K, Gałecka K, Trochanowska-Pauk N, Bohara R, et al. Near-infrared photobiomodulation of blood reversibly inhibits platelet reactivity and reduces hemolysis. Scientific reports. 2022; 12: 1-12.
- Araldi RP, de Melo TC, Mendes TB, de Sá Júnior PL, Nozima BHN, et al. Using the comet and micronucleus assays for genotoxicity studies: A review. Biomedicine & Pharmacotherapy. 2015; 72: 74-82.
- Zhou Q, Cui J, Liu Y, Gu L, Teng X, Tang Y. EGCG alleviated Mn exposure-caused carp kidney damage via trpm2-NLRP3-TNF-αJNK pathway: Oxidative stress, inflammation, and tight junction dysfunction. Fish & Shellfish Immunology. 2023; 134: 108582.
- Sumino K, Hayakawa K, Shibata T, Kitamura S. Heavy metals in normal Japanese tissues: amounts of 15 heavy metals in 30 subjects. Archives of Environmental Health: An International Journal. 1975; 30: 487-494.
- Dhapake PR, Avari JG. Application of polymeric nanoparticles in oral delivery of recombinant human erythropoietin: A review. Journal of Drug Delivery and Therapeutics. 2019; 9: 403-407.
- Singh SP, Kumari M, Kumari SI, Rahman MF, Mahboob M, et al. Toxicity assessment of manganese oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral exposure. Journal of Applied Toxicology. 2013; 33: 1165-1179.
- Huang P, Chen C, Wang H. et al. Manganese effects in the liver following subacute or subchronic manganese chloride exposure in rats Ecotoxicol Environ Saf. 2011; 74: 615-622.
- Mannem P. Protective effects of ginger extract against lead induced hepatotoxicity in male albino rats. IOSR J Environ Sci Toxicol Food Technol. 2014; 8: 52-9.
- Huang M, Wang Y, Song M, Chen F. Bimetallic CuCo Prussian blue analogue nanocubes induced chemiluminescence of luminol under alkaline solution for uric acid detection in human serum. Microchemical Journal. 2022; 181: 107667.
- Haddad Y, Vallerand D, Brault A, et al. Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid Based Complement Alternat Med. 2011.
- Jouve H, Melgar E, &, Lizárraga B. A study of the binding of Mn2+ to bovine pancreatic deoxyribonuclease I and to deoxyribonucleic acid by electron paramagnetic resonance. Journal of Biological Chemistry. 1975; 250: 6631-6635.
- Khan PK, Kesari VP, Kumar A. Mouse micronucleus assay as a surrogate to assess genotoxic potential of arsenic at its human reference dose. Chemosphere. 2013; 90993–997.
- Noshy MM, Hussien NA, &, El-Ghor AA. Evaluation of the role of the antioxidant silymarin in modulating the in vivo genotoxicity of the antiviral drug ribavirin in mice. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2013; 752: 14-20.