Review Article
Volume 2, Issue 7
Circulating Particles and Related Nucleic Acid as Highlighted Biomarker in Prostate Cancer
Elham Ghazizadeh1*; Mahya Sadeghi2; Elham mehri3
1Mashhad University of Medical Sciences, Iran.
2Faculty of Engineering Christian-Albrechts-Universität zu Kiel / Kiel University Kaiserstr. 2, 24143 Kiel, Germany.
3Islamic Azad University, Yazd, Iran.
4Faculty of Medicine, Gilan University of Medical Sciences, Iran.
Corresponding Author :
Elham Ghazizadeh
Email: elhamgenetic@yahoo.com
Received : Jun 01, 2023 Accepted : Jun 28, 2023 Published : Jul 05, 2023 Archived : www.meddiscoveries.org
Citation: Ghazizadeh E, Sadeghi M, Mehri E. Circulating Particles and Related Nucleic Acid as Highlighted Biomarker in Prostate Cancer. Med Discoveries. 2023; 2(7): 1051.
Copyright: © 2023 Ghazizadeh E. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Since prostate cancer (PC) is the second most malignancy in men, the accurate and timely detection is crucial. Presently the gold standard test in prostate cancer detection is about Prostate-specific antigen (PSA) screening. Although PSA monitoring correlated with reduction of PC mortality, but it is not accurate enough because of high false positive rate. Detection of circulating biomarkers as a novel approach have significantly advanced the diagnosis and also prognostic screening of PC. Many discoveries represent several types of circulating biomarkers such as circulating tumor cells (CTC), extracellular vesicles (EV), microparticles (MP), tumor-derived exosomes (TDE), prostasomes, and cell-free nucleic acids. In this review, we summarize the latest investigations for nominating and demonstrating body fluids biomarkers and also review the potential of using them for early detection or other clinical managements in the case of prostate cancer.
Keywords: Prostate cancer; Biomarker; Circulating biomarker; Prognosis.
Introduction
Based on the World Health Organisation (WHO) reports in 2020, prostate cancer is the third most common diagnosed malignancy. With 1,414,259 cases (7.3% of the total), prostate cancer is preceded only by lung and colorectal cancer with 2,206,771 and 1,148,515 cases respectively (11.4 and 10.0%) [1].
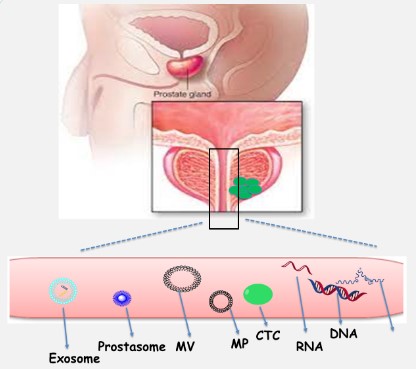
Prostate cancer is a progressive genetic and epigenetic abnormality in a solid organ that accounts for an important mortality factor in Europe and USA [2,3] and is known as the sixth cause of cancer-related death among men worldwide [4]. Regular screenings of the (PSA) through the control of metastasis can reduce the mortality rate [2]. Common screening methods include digital rectal examination, blood serum PSA test, and prostate biopsy, which have limitations and patients receive negative results after the prostate biopsy test. Regardless the role of PSA, the diagnosis of prostate cancer relies on transrectal or transperineal biopsy. For example; Magnetic resonance imaging (MRI) offers increasingly reliable visualization of potentially significant prostate cancers and thus has shown advantages as a means by which to better select patients for biopsy and facilitate direct targeting of lesions during biopsy. MRI also provides information for staging tumor extent and monitoring treatment response [5,6]. In addition, PSA screening for early diagnosis of the disease may cause unnecessary and harmful treatments despite such an advantage as the prevention of disease progression [4]. Not only, these limitations cause the inherent deficiency of this antigen, but also, they have diverted attentions to other new biomarkers in prostate cancer, which are found in the body fluids [7]. Novel biomarker-based molecular tests eliminate these limitations and reduce the number of unnecessary biopsies through increasing the specificity in the diagnosis. These tests are far better and more efficient for the detection of diseases than the PSA test alone [2]. Many biomarkers have been identified for the detection of this disorder, some of which are in tissues and some are circulating biomarkers (Figure 1). Some advantages of circulating biomarkers relative to tissue ones, which are more highlighted in this article, include easier and more availability at certain intervals and being nonaggressive or with lower aggressiveness [7]. This research tries to show about the circulating particles and related nucleic acid as new biomarkers in modified clinical prostate cancer diagnoses. These particles as Ev count (exosome, prostasome, micropariticles, apoptotic particles) and Tumour-derived cellfree molecules such as (ctDN, protein, ctRNA) are highlighted biomarkers in prostate cancers which differ fome the other circulating biomarkers as proteins, lipids, glycoproteins.
Extracellular vesicles (EV)
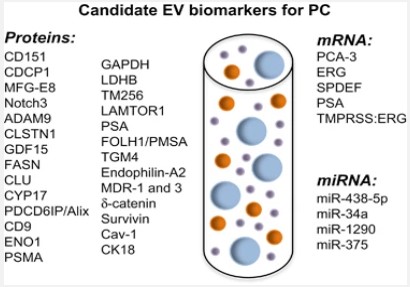
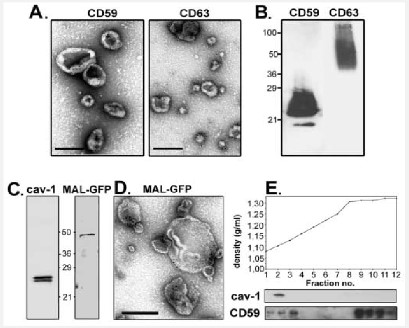
First introduced as “platelet dust” by Wolf, some of extracellular vesicles include micro-particles MP, micro-vesicles, and exosomes, which have today received a considerable attention for the diagnosis of many diseases. A characteristic of these particles is their size, with exosomes having a diameter of <100 nm whereas those of micro-particles MP and micro-vesicles range from 100 to 1000 nm [8]. Exosomes are formed through budding into secondary endosomes, but micro-vesicles bud and form towards the exterior plasma membrane [9,10]. In a plethora of studies, extracellular vesicles EVs have been used in the cancer diagnosis. The use of extracellular vesicles EV is a low-cost method with minimal aggression to recognize possible disease development in a person even prior to the initiation of signs and indications. The effect of tumor-derived extracellular vesicles EV on the initial formation of niche was demonstrated in an in vitro study, and it was also determined that these vesicles were effective in tumoral escape and aggression as well as in angiogenesis [8]. It should be noted that some mechanisms reduce the sensitivity of immune cells to cancer cells, including complement resistance that protects cancer cells against the immune system antibodies. This mechanism is done through vesicle shedding by the terminal components of the complement resulted from the plasma membrane. Another similar shedding, which reduces the susceptibility of T cell produced by the Fas apoptosis, arises from the Fas ligand on the surface of cancer cells [11]. There are other mechanisms, including phagocytosis, macropinocytosis, and particle confinement internally via membrane fusion and externally by contact with surface proteins, which stimulate the cells receiving extracellular vesicles EV [12]. Most studies have shown that PCA3, ERG, BIRC5, TMPRRS2 and TMPRRS2: ERG in urinary EV can differentiate between healthy and PCa patients. On the other hand, several studies have been designed to identify novel EV miRNAs for PCa diagnosis. miR-1246, miR-574 and miR-107 was found significantly altered in the serum of PCa patients. A follow-up study, including 240 individuals, showed that EV-derived PSA included PSA in a panel of 5 proteins (CD63–GLPK5–SPHM–PSA–PAPP) able to distinguish between low- and high-grade patients [84].
These miRNAs showed a similar behaviour in urinary EVs These vesicles have been also examined in PC in terms of difference; various release mechanisms, effects on cellular relations, and specific surface markers (Figure 2). Despite the importance of specific surface markers of these vesicles in the detection of a disease, some useful populations of micro-vesicles and microparticles MP have been disappeared due to limited studies in this field, and differences in the isolation protocols have restricted the evaluation and comparison of information [13].
MP/MV
Microparticles and microvesicles (MP/MV) have common characteristics with their parent cells; some characteristics of these biological molecules include mRNA, miRNA, surface receptors, and proteins of internal membranes [14,15]. Although there are limited investigations on the specific markers present on the surface of these vesicles, studies conducted among examined micro-particle MP populations have characterized some of diseases in human, such as hepatitis C, arthritis, and malaria, based on these micro-particles MP [16,17]. Several diseases and specific micro-particle MP populations were examined in these studies [16-19]. Results revealed that specific markers of MP/MV and their levels could be specific to each disease, and that some markers are common in most of diseases [20]. To predict disease characteristics based on MP/MV, a wide range of diseases can be evaluated by only a blood test with the least invasion, which is performed through standard isolation protocols and a surface MP/MV marker. It can, therefore, be stated that some disease characteristics are related to specific populations of MPs [8]. MVs have biological functions in the transfer of gene products, intercellular signaling, and intracellular connections [11], and their immune effects depend on the cell surface receptors and ligands [21]. After the conjugation of MVs with each other, their contained miRNA enters the target cell. In fact, MVs may cause epigenetic changes via genetic data transfer to target cells [22]. In a study by Ratajczak J et al., an epigenetic reprogramming was performed from stem/mature progenitor hemopoitic cells using micro-vesicles derived from murine fetal stem cells [23]. An easy screening method is to use the genetic material of MVs for the detection of cancer markers, which provides novel diagnostic information [24,25]. Tumoral and stromal cells are interconnected through MVs and these results in the growth, invasion, and spread of the tumor. Genetic analyses demonstrated that overexpression of miRNA in microvesicles derived from mesenchymal stem cells has an effect on the cellular survival and differentiation as well as the regulation of the immune system [11]. Micro-vesicles derived from cancer cells transfer specific proteins and RNAs to target cells and quantitative and qualitative information in the circulating MVs is obtained through proteomic and cytoflourometric analyses. For example, high levels of MV that cause the expression of CD63 and Caveolin-1 in the plasma of patients with melanoma [11], high levels of tissue factor and MUC1 in breast and pancreas cancers [26], high levels of PMP in prostate cancer [27], high levels of epcam and CD24 in ovary cancer, and high levels of EGFRV3 in glioblastoma. All these cases have been evaluated as a specific biomarker in the diagnosis of diseases. Micro-vesicles containing such genetic material as mRNA and microRNA provide an easy diagnostic method for cancer markers, and results of such studies and access to the molecular information of micro-vesicles will help researchers to achieve diagnostics and prognostics [11].
Diagnostic of MV/MP particles in prostate cancer
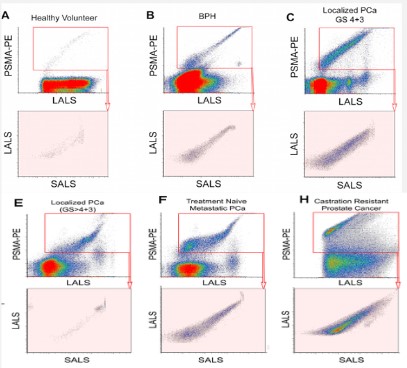
MP immunodetection assay is a cheap and fast method which easily performed to measure the levels of prostate biomarkers such as PSA in patients with prostate cancer and other urologic problems [28]. On the other side, Sanchez and his colleagues investigated that 2.36 × 106 EVs released from about 105 prostate cancer cells with an average of 1.4 EVs per cell per minute [29]. The large number of these diverse secreted EVs and the appropriate methods of examining them have made them valuable for diagnostic studies in cancers.Proteomics analysis showed that 16% of 266 PC-3 cell-released microvesicles’ proteins were classified as extracellular proteins that are promising biomarkers for prostate cancer and clinical validation studies in biological fluids [30]. Also, urinary MVs as a potential platform of non-invasive diagnostic biomarker differentiate patients with prostate cancer with 81% overall accuracy [31]. Distinguishing subpopulations of prostate extracellular vesicles in patient plasma, investigators used nanoscale flow cytometry. The results demonstrated that prostate derived EVs are primarily of cell membrane origin, MV/MP, and CD9 was the most abundant marker on them (12-19%) [32]. MVs can also play several roles in the progression of cancer due to their cargo. Prostate cancer-derived MVs express matrix metalloproteinases (MMP9 and MMP14) through stimulation of ERK1/2 phosphorylation in metastatic stages [33]. In the study of khorram and et al; was shown the relation between prostate microparticles (PMPs) in prostate cancer patients and also their relationship in prostate cancer metastasis stage [82] (Figure 3).
Exosomes
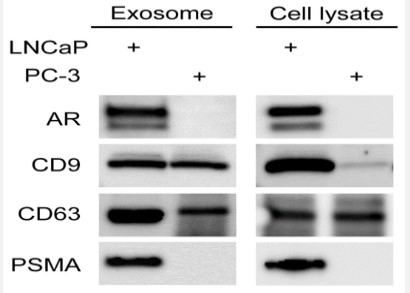
Another liquid biopsy biomarker is tumor-derived exosomes (TDE) which can provide significant advantages for various therapeutic and diagnostic approaches. Exosomes are a type of extracellular vesicle with a diameter in the range of 30–150 nm, secreted by most cell types of the body including cancer cells [43]. Since exosomes are permanently found in large amounts in all types of extracellular fluids such as urine, saliva, cerebrospinal fluid, and blood; they are a good option for cancer diagnosis. Exosomes are released by the exocytosis of multivesicular bodies (MVBs) which are produced by a process of endosomal membranes inward budding [44]. Like CTCs, exosomes are identified by their surface markers (e.g., tetraspanin CD63, CD81, CD82, CD53 and CD37, heat shock proteins, lysosomal proteins, flotillin, Annexin) and they are able to transfer a broad collection of cargo such as DNAs, RNA, proteins and lipids. Particularly, recent studies suggest that TDE are important enhancers of prostate cells survival, proliferation, invasion, and angiogenesis through their cargo [45,46]. The presence of some prostate cancer related proteins and mRNA such as Prostate Specific Membrane Antigen (PSMA), Prostate Stem Cell Antigen (PSCA), and Prostate Cancer Antigen-3 (PCA-3) mRNA were reported in PC-derived exosomes and absolutely could be informative[47]. Interestingly, Webber JP et al. demonstrate that prostate cancer cells derived exosomes can present transforming growth factor-β (TGF-β) to fibroblasts and as a result these cells transform to Carcinoma-associated fibroblasts (CAFs) (Figure 4) [48]. Several types of RNA including non-coding RNAs are carried by the exosomal compartments. TDE-delivered microRNAs (miRNAs) level can be correlated with the degree and stage of cancer progression. For example, exosomal miR-143 can inhibit prostate cancer progression [49], and the level of miR-141 is elevated in serum samples of patients with prostate cancer [50]. Another study precisely introduced a panel of several plasma samples’ miRNAs from 82 PC patients. According to the results of this study, the combination of miR-20a, -21, -145, and -221 can potentially characterize PC patients with low risk of aggressiveness from those with high risk [51]. In addition, urinederived exosomes could be use as diagnostic biomarkers for prostate cancer. Remarkably, the significantly higher expression of PSA and PCA3 mRNA in PSMA-positive exosomes of patients with PC, was reported by Ping Li et al [52]. Moreover, these RNAbased contents may play a role as biomarkers for PC. In most studies there is no discrimination between free circulating miRNAs and miRNAs in EVs. Recent studies have suggested some panels of EVs-associated miRNAs in blood of PC patients. Bryant et al. have found that miR-141 and miR-375 were expressed significantly higher in the plasma-derived exosomes of prostate cancer patients compared with controls [53]. In the other study, the levels of miR-151a-5p, miR-204-5p, miR-222-3p, miR-23b-3p and miR-331-3p in exosome enriched cell-free urine samples from PC individuals evaluated in combination with the serum PSA test. This novel multimarker model could potentially be used for prediction of PC recurrence after prostatectomy [54].
Prostasomes
In addition to exosomes, there are other small membranecoated particles that are commonly found in semen, which secreted by prostate epithelial cells into the lumen of the prostatic ductal system. More than 30 years ago, Ronquist and his colleagues identified these nanosized objects and named them prostasome [55]. Although prostasomes and exosomes are formed and released in the following of MVE fusion with the plasma membrane of the cell, they differ in size and appearance [56]. Prostasomes are heterogeneous as regards size and the diameter of them is between 50 nm and 0.5 µm. Many studies have shown that prostasomes are present in the plasma, semen, and urine of PC patients (Figure 5) [57,58]. Normally, prostasomes seem to play a critical role in the process of reproduction by affecting on sperm motility and capacitation. In addition, they seem to be involved in postponement of acrosomal reaction until the sperm reach the egg, as well as sperm protection from female’s immunological attacks. The interaction of prostasomes with the local female immune system including immunomodulatory skills exerted by prostasomal small RNA biotype. prostasomes may be a candidate antigen for Antisperm Antibodies (ASA) and we raised polyclonal chicken antibodies against purified seminal prostasomes. Prostasomes could also present antibacterial effects and antioxidant capacities. It was demonstrated that prostasomes indeed reduced ROS production by sperm preparations containing polymorphonuclear neutrophils. Prostasomes can well serve as a reservoir of this precursor of the antibiotic peptide LL-37. So, prostasomes may exert antibacterial activities by more than one route [59]. On the other side, investigators suggested that prostasomes also involved in prostate cancer initiation and progression through their physiological features. Indeed, besides normal prostate cells, prostasomes are released from neoplastic and metastasis prostate cancer cells [60]. Like other EVs, prostasomes are contained cancer related cargo, including oncoproteins, oncomiR, and mutant transcripts. Therefore, they can be a feasible biomarker for PC [57-61]. Interestingly, Kato T, et al. showed that prostasome levels in the blood of PC patients were higher than healthy individuals [62], and it has also been suggested that prostasomes concentration correlates with disease severity [57]. For instance, Phosphatase and tensin homolog (PTEN) as a tumor suppressor protein and Survivin as an antiapoptotic factor were significantly higher in prostasomes isolated from blood of PC patients in comparison with healthy subjects [63,64]. It seems that prostasomes isolated from urine are more numerous than blood and they present hundreds of proteins like PMSA, PSA, prostatic acid phosphatase, and prostate transglutaminase [65]. Another interesting point is that prostasomes have shown epigenetic effects by reserving and transferring mRNA and non-coding RNAs.
Circulating tumor cells as prostate circulating biomarker
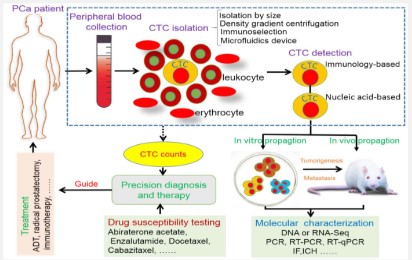
CTCs are rare tumor cells which separated from primary tumor cells and shed into the peripheral blood of patients with a variety of metastatic malignancies, including prostate cancer [34]. The frequency of them estimated to 1 CTC in 106–9 blood cells [35], thus because of this rarity, the clinical use of CTCs is limited. CTCs provide a qualified biomarker with valuable real-time information which can use for early cancer detection, prognosis, response indication, and treatment effectiveness. In the most of the existing technologies, isolated cells are detected accurately by various methods such as immunostaining, visual microscopy, biomechanical discrimination and polymerase chain reaction (PCR) [36]. Today, a variety of successful devices and technologies have been developed to harness the potential of CTCs based on molecular properties of them, like Cell search device that uses magnetic beads labeled with epithelial cell–adhesion molecule (EpCAM) antibodies to isolate tumor cells [37]. Accordingly, generic labels can be used for detection these cells include groups such as epithelial cell–specific antigens (CK8, CK18, or CK19), epithelial cell–adhesion molecule (EpCAM) or organ-specific antigens (prostate-specific antigen [PSA], prostate-specific membrane antigen [PSMA]), carcinoembryonic antigen, and human epidermal growth factor receptor [38]. The association of CTC count and survival in patients with metastatic prostate cancer has been shown in several studies. CTCs were present in 21% of patients with localized prostate cancer [39] . The number of CTCs in metastatic PC was significantly elevated in comparison to non-metastatic. Also, existence of bone and visceral lesions demonstrated the highest significant median CTC count, whereas individuals with soft tissue metastatic PC, displayed CTC counts approximately equal to the control group [40]. Choi et al. also reported that EpCAM+ CTCs was significantly higher in patients with prostate cancer than in healthy volunteers [40]. Additionally, the number of CTCs in patients with metastatic castration-resistant prostate cancer, Increased during the first 12 weeks of treatment. Moreover, this investigation reported that in patients treated with abiraterone + prednisone, corticosteroids alone, and chemotherapy, CTCs declined 30% in association with longer overall survival [41]. Since, CTCs can be used in association with other procedures as metastatic cancer biomarker; Okegawa et al. examined the surface EGFR expression levels in the prognostic and therapeutic value of CTCs before docetaxel chemotherapy. The results showed that 40.5% of prostate cancer patients were positive for EGFR in CTCs and had a shorter overall survival than patients with EGFR-negative CTCs [42].
Circulating cell-free DNA (cfDNA) or RNA (cfRNA) in PC
cfDNA shows as DNA parts released in blood by typical and tumor cells. Strikingly, DNA discharged by tumor cells represents to a little division of cfDNA, called ctDNA, which appears a littler estimate than cfDNA discharged by typical cells. From a prognostic point of see, ctDNA concentration in blood seem possibly be complementary to PSA tests or supplant it. Tall ctDNA concentration, without a doubt, relates with destitute PCa outcom [66,67]. The advanced arrange PCa patients have a better ctDNA concentration compared to those with localized malady or solid controls. In this think about, ctDNA was quantifiedwith a Qubit 3.0 fluorometer and a DNA dsDNAHS Measure Unit [68].
Table 1: Specific mRNA markers for detection of prostate cancer circulating tumor cells.
| mRNA markers | (Refs.) |
|---|---|
| PSA | (72,73) |
| PSMA | (72) |
| PCA3 | (75) |
| PSCA | (69) |
| AR | (74) |
| AR-V7 | (76) |
| TMPRSS2-ERG | (63,75) |
| KLK2 | (69) |
| KLK3 | (69,75) |
According to cfDNA level modification as a clinical biomarker in PCa patients, Cell free DNA concentration was relatively higher in PC patients in comparison to persons with benign prostatic hyperplasia (Table 2) [80]. Investigations suggested that the levels of total cell free DNA in the plasma of metastatic PC patients with higher cell free DNA level had worse outcomes on hormonal therapy [81]. ctDNA examination could represent a substantial cost-effective elective to tissue biomarkers examination progressed organize PCa. Interests, this approach may well be valuable to recognize prescient biomarkers that can be assist surveyed in future clinical trials [69,70]. Similarly to DNA fragments, tumor cells shade RNA-derived fragments in blood, known as circulating tumor RNA (ctRNA), ctRNA- messenger RNA (mRNA), microRNA(miRNA) and long non-coding RNA, may similarly represent a fascinating biosource for molecular analysis [71]. In particular, the miRNAs expression profiling analysis is increasing to perform diagnosis, staging, progression, prognosis and treatment response [72,73]. miRNA can be extracted from ribonucleoprotein complexes or EV.The importance of research on extracellular biomarkers, as an important marker in cancer detection and progression in cancerous patients, can be realized by the fact that these genetic material in the forms of mRNA and miRNA were in the vesicles. MiRNAs are small noncoding RNA molecules that contain 19-25 nucleotides and are involved in the regulation of gene expression. Diverse miRNAs are expressed in different cancers and examining the characteristics of these miRNAs is associated with the formation, development, and response of the tumor to treatments. Some examples are presented in the following:
In patients with gastric cancer, breast cancer, and glioblastoma, cancer-specific mRNA was identified and expressed in extracellular vesicles [71-73]. miRNA can also be released from the cells and participate in the cycle, or be captured by other cells. A stable miRNA in the cycle is the one that attaches to RNA-binding proteins or dense lipoproteins, or is encapsulated into extracellular vesicles [67]. Other than miRNAs, components associated with extracellular vesicles, such as proteins and long, non-coding RNAs (lncRNAs), may be a potential biomarker [67]. LncRNAs are non-coding transcripts in human genome being involved in the pathogenesis of different diseases, which are expressed in a variety of human cancers. Most of lncRNAs are used as a diagnostic and indicator factor for the detection of diseases and cancers. For instance, exosomal lincRNA-p21 levels was higher in the patients with PC, thus its exosomal level can help to improve the diagnostic prediction of the prostate malignant state [74]. These studies help us to obtain information about molecular compositions of micro-vesicles and consequently to develop diagnostic modalities [11]. To illustrate, PCA3, GOLPH2, and SPINK1 transcript have been detected in the urine of PC [75,76]. In the study conducted by Donovan, the co-expression of EGR and PCA3 was identified as a possible biomarker [77].
DNA and RNA abnormalities in blood could be used to better detection of PC, in fact, in the patients with prostate cancer, tumor DNA and RNA shed into the bloodstream [78,79].
Table 2: Association between increase in cfDNA concentration (5 ng/mL) or in cfDNA fragment size (5 bp) and prostate cancer status.
| Crude | Adjusted | |||||
|---|---|---|---|---|---|---|
| cfDNA measure | Prostate cancer status | Variable | Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value |
| Localized vs healthy | dDNA concentration | 1.10 (0.82-1.61) | 0.64 | 1.05 (0.77- 1.69)* | 0.72 | |
| Age | - | - | 1.54 (1.10- 2.10) | 0.01 | ||
| mCRPC | dDNA concentration | 1.93 (1.34-3.18) | 00025 | 1.69 (1.16- 2.93)* | 0.034 | |
| dDNA concentration (5 ng/mL.) | vs healthy | Age | - | - | 2.39 (1.69- 3.43) | 1E-06 |
| dDNA concentration | 1.47 (1.22-2.01) | 00017 | 1.34 (1.05- 1.76) | 0.027 | ||
| mCRPC vs localized | Age | - | - | 1.76 (1.28- 2.60) | 0.002 | |
| PSA | - | - | 1.54 (1.22- 2.01) | 0.0008 | ||
| dDNA fragment size (5 bp) | Localized vs healthy | dDNA Fragment size | 0.86 (0.73-0.9) | 0.003 | 0.77 (0.66- 0.90)* | 0.0008 |
| Age | - | - | 1.61 (1.22- 2.10) | 0.001 |
Conclusion
Prostate cancer diagnosis through biomarkers is still controversial, because of the accurate biomarkers panel absence. PSA was introduce as a strong PC biomarker more than two decades’ ego, but nowadays it has been made clear that the use of PSA as a biomarker has not been able to reduce mortality from prostate cancer.
Other new biomarkers have introduced after identifying and introducing blood based biomarkers. Using high throughput molecular screening of CTCs, MVs, MPs, EVs, TDEs, and prostasomes might lead to find the combination of specific biomarkers that can accurately diagnose PC and other malignancies. These liquid biopsy biomarkers show the potential to gain comprehensive information on PCa genetic landscape, and give information about the metastatic sites. Liquid biopsy for detection these particles could guide therapeutic decisions and accelerate the development of precision medicine in PCa. Despite the assurance of these powerful tests with high throughput, there is still no routine use of these particles for cancer in the early stages, and their widespread use has been limited due to the high cost and lack of skills of trained people. As a result, it is suggested to study more researches in the field of standardization and design of less expensive methods. Although also, several progresses have been placed into investigating novel diagnostic and prognostic biomarkers for PCa, considering the inability of current biomarkers to predict disease aggressiveness, new efforts are needed to paint the intriguing PCa picture.
References
- Rothwax, J.T., et al., Multiparametric MRI in biopsy guidance for prostate cancer: fusion-guided. BioMed research international, 2014. 2014.
- Gandaglia, G., et al., Structured population-based prostatespecific antigen screening for prostate cancer: the European Association of Urology position in 2019. European urology, 2019. 76(2): p. 142-150.
- Haluskova, J., L. Lachvac, and V. Nagy, The investigation of GSTP1, APC and RASSF1 gene promoter hypermethylation in urine DNA of prostate-diseased patients. Bratislavske lekarske listy, 2015. 116(2): p. 79.
- Birkeland, S., et al., Men’s view on participation in decisions about prostate-specific antigen (PSA) screening: patient and public involvement in development of a survey. BMC medical informatics and decision making, 2020. 20: p. 1-10.
- Descotes, J.-L., Diagnosis of prostate cancer. Asian journal of urology, 2019. 6(2): p. 129-136.
- Gann, P.H., et al., Risk factors for prostate cancer detection after a negative biopsy: a novel multivariable longitudinal approach. Journal of Clinical Oncology, 2010. 28(10): p. 1714.
- Batra, J.S., S. Girdhani, and L. Hlatky, A quest to identify prostate cancer circulating biomarkers with a bench-to-bedside potential. Journal of biomarkers, 2014. 2014.
- Julich, H., et al., Extracellular vesicle profiling and their use as potential disease specific biomarker. Frontiers in immunology, 2014. 5: p. 413.
- Théry, C., L. Zitvogel, and S. Amigorena, Exosomes: composition, biogenesis and function. Nature reviews immunology, 2002. 2(8): p. 569-579.
- Record, M., et al., Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2014. 1841(1): p. 108-120.
- Camussi, G., et al., Exosome/microvesicle-mediated epigenetic reprogramming of cells. American journal of cancer research, 2011. 1(1): p. 98.
- Mulcahy, L.A., R.C. Pink, and D.R.F. Carter, Routes and mechanisms of extracellular vesicle uptake. Journal of extracellular vesicles, 2014. 3(1): p. 24641.
- Monguio-Tortajada, M., et al., Extracellular-Vesicle Isolation from Different Biological Fluids by Size-Exclusion Chromatography. Curr Protoc Stem Cell Biol, 2019. 49(1): p. e82.
- Dziechciowski, M., et al., Diagnostic and prognostic relevance of microparticles in peripheral and uterine blood of patients with endometrial cancer. Ginekol Pol, 2018. 89(12): p. 682-687.
- Mege, D., L. Panicot-Dubois, and C. Dubois, Tumor-Derived Microparticles to Monitor Colorectal Cancer Evolution. Methods Mol Biol, 2018. 1765: p. 271-277.
- Mahajan, S., et al., Comparative evaluation of three rapid immunochromatographic test assays with chemiluminescent microparticle immunoassay for the detection of hepatitis C virus antibody. Virusdisease, 2019. 30(3): p. 373-379.
- Tiberti, N., et al., Exploring experimental cerebral malaria pathogenesis through the characterisation of host-derived plasma microparticle protein content. Sci Rep, 2016. 6: p. 37871.
- Wadood, M. and M. Usman, Comparative Analysis of Electrochemiluminescence Assay and Chemiluminescent Microparticle Immunoassay for the Screening of Hepatitis C. Indian J Hematol Blood Transfus, 2019. 35(1): p. 131-136.
- Morota, K., et al., A new sensitive and automated chemiluminescent microparticle immunoassay for quantitative determination of hepatitis C virus core antigen. J Virol Methods, 2009. 157(1): p. 8-14.
- Harel, M. and T. Geiger, Plasma Biomarker Identification and Quantification by Microparticle Proteomics. Methods Mol Biol, 2017. 1619: p. 477-486.
- Ratajczak, J., et al., Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia, 2006. 20(5): p. 847-856.
- Camussi, G., et al., Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res, 2011. 1(1): p. 98-110.
- Shin, D.M., et al., Molecular signature of adult bone marrowpurified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia, 2010. 24(8): p. 1450-61.
- Miranda, K.C., et al., Massively parallel sequencing of human urinary exosome/microvesicle RNA reveals a predominance of non-coding RNA. PLoS One, 2014. 9(5): p. e96094.
- Ben-Dov, I.Z., et al., Cell and Microvesicle Urine microRNA Deep Sequencing Profiles from Healthy Individuals: Observations with Potential Impact on Biomarker Studies. PLoS One, 2016. 11(1): p. e0147249.
- Giusti, I., S. D’Ascenzo, and V. Dolo, Microvesicles as potential ovarian cancer biomarkers. BioMed research international, 2013. 2013.
- Dashevsky, O., D. Varon, and A. Brill, Platelet‐derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP‐2 production. International journal of cancer, 2009. 124(8): p. 1773-1777.
- Sanchez de la Muela, P., et al., Microparticle immunoenzymatic assay for detection of prostate specific antigen: characterization of the technique and comparative analysis with a monoclonal immunoradiometric assay. Mil Med, 1995. 160(8): p. 416-9.
- Stratton, D., et al., Label-free real-time acoustic sensing of microvesicle release from prostate cancer (PC3) cells using a Quartz Crystal Microbalance. Biochem Biophys Res Commun, 2014. 453(3): p. 619-24.
- Sandvig, K. and A. Llorente, Proteomic analysis of microvesicles released by the human prostate cancer cell line PC-3. Mol Cell Proteomics, 2012. 11(7): p. M111 012914.
- Motamedinia, P., et al., Urine Exosomes for Non-Invasive Assessment of Gene Expression and Mutations of Prostate Cancer. PLoS One, 2016. 11(5): p. e0154507.
- Padda, R.S., et al., Nanoscale flow cytometry to distinguish subpopulations of prostate extracellular vesicles in patient plasma. Prostate, 2019. 79(6): p. 592-603.
- Castellana, D., et al., Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res, 2009. 69(3): p. 785-93.
- Cattrini, C., et al., Role of circulating tumor cells (CTC), androgen receptor full length (AR-FL) and androgen receptor splice variant 7 (AR-V7) in a prospective cohort of castration-resistant metastatic prostate cancer patients. Cancers, 2019. 11(9): p. 1365.
- Cristofanilli, M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. in Seminars in oncology. 2006. Elsevier.
- Alix-Panabières, C. and K. Pantel, Circulating tumor cells: liquid biopsy of cancer. Clinical chemistry, 2013. 59(1): p. 110-118.
- Adams, D.L., et al., Cytometric characterization of circulating tumor cells captured by microfiltration and their correlation to the cellsearch® CTC test. Cytometry Part A, 2015. 87(2): p. 137-144.
- Ried, K., et al., New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers. Frontiers in Oncology, 2020. 10: p. 582.
- Davis, J.W., et al., Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: initial results in early prostate cancer. The Journal of urology, 2008. 179(6): p. 2187-2191.
- Thalgott, M., et al., Detection of circulating tumor cells in different stages of prostate cancer. Journal of cancer research and clinical oncology, 2013. 139(5): p. 755-763.
- Lorente, D., et al., Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann Oncol, 2018. 29(7): p. 1554-1560.
- Okegawa, T., et al., Epidermal Growth Factor Receptor Status in Circulating Tumor Cells as a Predictive Biomarker of Sensitivity in Castration-Resistant Prostate Cancer Patients Treated with Docetaxel Chemotherapy. Int J Mol Sci, 2016. 17(12).
- Czystowska-Kuzmicz, M. and T.L. Whiteside, The potential role of tumor-derived exosomes in diagnosis, prognosis and response to therapy in cancer. Expert opinion on biological therapy, 2020.
- Ludwig, N., et al., Isolation and Analysis of Tumor-Derived Exosomes. Current protocols in immunology, 2019. 127(1): p. e91.
- Liu, C.M., et al., Exosomes from the tumor microenvironment as reciprocal regulators that enhance prostate cancer progression. International Journal of Urology, 2016. 23(9): p. 734-744.
- Koga, K., et al., Purification, characterization and biological significance of tumor-derived exosomes. Anticancer research, 2005. 25(6A): p. 3703-3707.
- Liu, T., D.E. Mendes, and C.E. Berkman, Functional prostate-specific membrane antigen is enriched in exosomes from prostate cancer cells. International journal of oncology, 2014. 44(3): p. 918-922.
- Webber, J.P., et al., Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene, 2015. 34(3): p. 290-302.
- Che, Y., et al., Exosomes derived from miR-143-overexpressing MSCs inhibit cell migration and invasion in human prostate cancer by downregulating TFF3. Molecular Therapy-Nucleic Acids, 2019. 18: p. 232-244.
- Samsonov, R., et al., Lectin‐induced agglutination method of urinary exosomes isolation followed by mi-RNA analysis: application for prostate cancer diagnostic. The Prostate, 2016. 76(1): p. 68-79.
- Shen, J., et al., Dysregulation of circulating microRNAs and prediction of aggressive prostate cancer. The Prostate, 2012. 72(13): p. 1469-1477.
- Tommasi, S., et al., Standardization of CTC AR-V7 PCR assay and evaluation of its role in castration resistant prostate cancer progression. Prostate, 2019. 79(1): p. 54-61.
- Bryant, R.J., et al., Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer, 2012. 106(4): p. 768-74.
- Fredsoe, J., et al., A five-microRNA model (pCaP) for predicting prostate cancer aggressiveness using cell-free urine. Int J Cancer, 2019. 145(9): p. 2558-2567.
- Stegmayr, B. and G. Ronquist, Promotive effect on human sperm progressive motility by prostasomes. Urological research, 1982. 10(5): p. 253-257.
- Ronquist, G.K., et al., Prostasomes are heterogeneous regarding size and appearance but affiliated to one DNA‐containing exosome family. The Prostate, 2012. 72(16): p. 1736-1745.
- Tavoosidana, G., et al., Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proceedings of the National Academy of Sciences, 2011. 108(21): p. 8809-8814.
- Rodríguez, M., et al., Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Molecular cancer, 2017. 16(1): p. 156.
- Vickram, A., et al., Human prostasomes an extracellular vesicle–Biomarkers for male infertility and prostrate cancer: The journey from identification to current knowledge. International journal of biological macromolecules, 2020. 146: p. 946-958.
- Gomà, A., et al., Multidrug resistance protein 1 localization in lipid raft domains and prostasomes in prostate cancer cell lines. OncoTargets and therapy, 2014. 7: p. 2215.
- Zijlstra, C. and W. Stoorvogel, Prostasomes as a source of diagnostic biomarkers for prostate cancer. The Journal of clinical investigation, 2016. 126(4): p. 1144-1151.
- Kato, T., et al. Serum exosomal P-glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. in Urologic Oncology: Seminars and Original Investigations. 2015. Elsevier.
- Gabriel, K., et al., Regulation of the tumor suppressor PTEN through exosomes: a diagnostic potential for prostate cancer. PloS one, 2013. 8(7): p. e70047.
- Khan, S., et al., Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PloS one, 2012. 7(10): p. e46737.
- Nilsson, J., et al., Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. British journal of cancer, 2009. 100(10): p. 1603-1607.
- Vaccaro, F., A. Roosendaal, and C. Iselin, [Early detection of prostate cancer: a summary of the past 10 years]. Rev Med Suisse, 2020. 16(717): p. 2330-2333.
- Matsuzaki, J. and T. Ochiya, Circulating microRNAs and extracellular vesicles as potential cancer biomarkers: a systematic review. International journal of clinical oncology, 2017. 22(3): p. 413-420.
- Woo, J., et al., Urine Extracellular Vesicle GATA2 mRNA Discriminates Biopsy Result in Men with Suspicion of Prostate Cancer. J Urol, 2020. 204(4): p. 691-700.
- Urabe, F., et al., miR-26a regulates extracellular vesicle secretion from prostate cancer cells via targeting SHC4, PFDN4, and CHORDC1. Sci Adv, 2020. 6(18): p. eaay3051.
- Urabe, F., et al., The miR-1908/SRM regulatory axis contributes to extracellular vesicle secretion in prostate cancer. Cancer Sci, 2020. 111(9): p. 3258-3267.
- Rezaei, M., et al., Extracellular Vesicle Transfer from Endothelial Cells Drives VE-Cadherin Expression in Breast Cancer Cells, Thereby Causing Heterotypic Cell Contacts. Cancers (Basel), 2020. 12(8).
- Kong, W., et al., Extracellular vesicle derived miR-544 downregulates expression of tumor suppressor promyelocytic leukemia zinc finger resulting in increased peritoneal metastasis in gastric cancer. Aging (Albany NY), 2020. 12.
- Jones, J., Glioblastoma single extracellular vesicle analysis profiles: wading into new oceans of tumor data. Neuro Oncol, 2019. 21(5): p. 562-564.
- Isin, M., et al., Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet, 2015. 6: p. 168.
- Roobol, M.J., A. Haese, and A. Bjartell, Tumour markers in prostate cancer III: biomarkers in urine. Acta Oncol, 2011. 50 Suppl 1: p. 85-9.
- Laxman, B., et al., A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res, 2008. 68(3): p. 645-9.
- McKiernan, J., et al., A urine-based Exosomal gene expression test stratifies risk of high-grade prostate Cancer in men with prior negative prostate biopsy undergoing repeat biopsy. BMC Urol, 2020. 20(1): p. 138.
- Fettke, H., et al., Combined Cell-free DNA and RNA Profiling of the Androgen Receptor: Clinical Utility of a Novel Multianalyte Liquid Biopsy Assay for Metastatic Prostate Cancer. Eur Urol, 2020. 78(2): p. 173-180.
- Vandekerkhove, G., et al., Circulating Tumor DNA Abundance and Potential Utility in De Novo Metastatic Prostate Cancer. Eur Urol, 2019. 75(4): p. 667-675.
- Condappa, A., et al., Evaluation of Plasma Circulating Cell Free DNA Concentration and Integrity in Patients with Prostate Cancer in Jamaica: A Preliminary Study. Diseases, 2020. 83.
- Fettke, H., et al., Prognostic Impact of Total Plasma Cell-free DNA Concentration in Androgen Receptor Pathway Inhibitor-treated Metastatic Castration-resistant Prostate Cancer. Eur Urol Focus, 2020.
- Garrastacho , M., et al., Extracellular vesicles as a source of prostate cancer biomarkers in liquid biopsies: a decade of research: British Journal of Cancer volume 126, pages331–350 (2022).