Research Article
Volume 2, Issue 6
Visceral Adiposity and Lipid Accumulation Product Indices as Valuable Predictors of Metabolic Syndrome in the Elderly: Bushehr Elderly Health (BEH) Program
Maryam Marzban1; Kamyar Asadipooya2; Mohamad Gholizade3; Iraj Nabipour4; Afshin Ostovar5; Bagher Larijani4; Akram Farhadi3; Amir Hossein Darabi3; Mohamad Hadi Emamat1; Fateme Mozafari3; Mohammdreza Kalantarhormozi3*
1Clinical Research Development Center, the Persian Gulf Martyrs Bushehr University of Medical Sciences, Bushehr, Iran.
2Division of Endocrinology and Metabolism, Department of Medicine, University of Kentucky, Kentucky, KY, USA 40504.
3The Persian Gulf Tropical Medicine Research Center, The Persian Gulf Biomedical Sciences Research Institute, Bushehr University
of Medical Sciences, Bushehr, Iran.
4The Persian Gulf Marine Biotechnology Research Center, the Persian Gulf Biomedical Sciences Research Institute, Bushehr Univer-
sity of Medical Sciences, Bushehr, Iran.
5Osteoporosis Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences,
Tehran, Iran.
Corresponding Author :
Mohammadreza Kalantarhormozi
Email: m.kalantarhormozi111@yahoo.com
Received : May 30, 2023 Accepted : Jun 20, 2023 Published : Jun 27, 2023 Archived : www.meddiscoveries.org
Citation: Marzban M, Asadipooya K, Gholizade M, Nabipour I, Kalantarhormozi M. Visceral Adiposity and Lipid Accumulation Product Indices as Valuable Predictors of Metabolic Syndrome in the Elderly: Bushehr Elderly Health (BEH) Program. Med Discoveries. 2023; 2(6): 1049.
Copyright: © 2023 Kalantarhormozi M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Metabolic Syndrome (MetS) encompasses a cluster of cardiometabolic risk factors and plays a significant role in elderly patients’ long-term complications. Herein, we studied the association of Lipid Accumulation Product (LAP) and Visceral Adiposity Index (VAI) with MetS to assess their validity for diagnosing and predicting MetS. In this cross-sectional study based on Bushehr elderly health program, data of 3000 old (more than 60) enrollees of phase 1 and 2426 of phase 2 of this cohort were evaluated. The adiposity indices of VAI, and LAP were measured. Simple regression analysis and simultaneously multivariable logistic regression were done. The sensitivity and specificity of LAP and VAI in predicting the development of MetS were also calculated. VAI and LAP had a strong association with MetS and could predict and had better sensitivity and specificity for detecting MetS compared to the other MetS risk factors. The incidence ratio for VAI >1.88 was 2.55 (95% CI, 2.26 to 2.87), and for LAP>37.10 was 2.45 (95% CI, 2.13 to 2.82). This study shows that LAP and VAI can be reliable indices to assess MetS in the elderly.
Keywords: Metabolic syndrome; Lipid accumulation product; Visceral adiposity index; Obesity; Elderly.
Introduction
In various societies, the increase in the elderly population and metabolic age-related disorders, such as cardiovascular diseases (CVDs), type 2 diabetes and etc., have a heavy economic and health burden at the national and international levels [1,2]. Metabolic Syndrome (MetS) and its components, including impaired glucose tolerance, central obesity, hypertension, and dyslipidemia, are associated with increased risk of metabolic disorders such as CVDs, cerebrovascular disease (CVA), and T2DM, through metabolic and inflammatory events [3,4].
Adipose tissue is a metabolically dynamic organ responsible for storing excess energy and capable of producing adipokines and proinflammatory cytokines that regulates metabolic homeostasis [5]. Adipose tissue accumulates in the body in two forms, subcutaneous and visceral [6]. Visceral adipose tissue that is located in the abdominal cavity surrounding the organs, is considered a major risk factor for cardio-metabolic disorders, independently of general adiposity [7,8]. In fact, central obesity is partially indicative of visceral fat, and the use of indicators related to visceral fat and Waist Circumference (WC) can be helpful in identifying high-risk populations in terms of metabolic diseases [9].
The increased attention to the Visceral Adipose Tissue (VAT) highlights the importance of accurate assessment of body fat components in the population studies. The current methods of assessing body composition, such as hydrostatic weighing, air displacement plethysmography, and bioelectrical impedance analysis, are not sensitive for measuring regional fat, especially visceral fat. On the other hand, the advanced methods to measure visceral adiposity, including Dual-Energy X-Ray Absorptiometry (DXA), Computed Tomography (CT), and Magnetic Resonance Imaging (MRI), are expensive and time-consuming and cannot be used in epidemiological screenings [10]. Visceral Adiposity Index (VAI) is a relatively well-known method that includes multiple variables to combine functional (serum Triglycerides [TG] and High-Density Lipoprotein [HDL] cholesterol levels) and anthropometric (Waist Circumference [WC], Body Mass Index [BMI]) parameters. VAI is a reasonably precise measurement for metabolic derangements, reflecting visceral adipose dysfunction but not directly visceral adiposity [11]. VAI is a predictor for T2DM insulin resistance and is associated with hyperinsulinemia, CVD, CVA, atherosclerosis severity and hyperandrogenism [12-15]. Also, few studies in the past have introduced this index as a good predictor of MetS [16,17]. Furthermore, Lipid Accumulation Product (LAP) index, which is calculated based on WC and TG [18], associated with insulin resistance, T2DM [19,20], metabolic syndrome, hepatic steatosis [21,22], CVD [18,23,24], hypertension [25], chronic kidney disease [26], and androgenicity [27]. Some studies have shown that LAP is a suitable indicator for the risk of CVD [28] and MetS [29] in the elderly population.
Present study based on data of Bushehr Elderly Health (BEH) Program can provide more accurate information in terms of incidence, causes, and prognosis determination according to the evaluation of a large populations and assessing them in a longer period of time. Therefore, our aim in the current study was to investigate the association of VAI and LAP indices with MetS incidence rate in a large elderly cohort population, so that if the results of previous studies are confirmed and by determining the optimal cut-off, we will contribute to the clinical applicability of these indicators for elderly people.
Materials and methods
Study design and population
We have previously reported the details of the studies design have been conducted in the Bushehr Elderly Health (BEH) program [1,2]. As a brief reminder, it was a prospective cohort study investigating the prevalence and risk factors of non-communicable diseases, including MetS, T2DM and CVDs in men and women older than 60 years living in Bushehr city of Iran. We selected the participants through a random sampling after receiving the invitation or self-referral to the research center for further evaluations. At the Persian Gulf Tropical Medicine Research Centre, a trained nurse recorded the demographic, medical, social, medication, biochemical and clinical data. The baseline measurements of 3000 enrollees were collected in the first phase between March 2013 and October 2014. The second phase of data collection of the same population, but 2,426 persons of them (80.87% response rate), lasted 2.5 years starting October 2015, and 574 participants could not participate in the study due to death, migration, or unwillingness to continue participation.
The inclusion criteria were age ≥60 years, signed written informed consent, residency in Bushehr city for at least one year ago, resident of Bushehr at least until the next 2 year, and generally acceptable mental and physical conditions.
Individuals who had BMI <18.5, chronic diseases, hematological and rheumatic diseases, inflammatory bowel diseases, chronic renal failure, Cushing syndrome, hyperthyroidism, hypothyroidism, history of cancer, excess alcohol consumption, drug abuse, and hospitalization in the previous two months, were excluded from the study.
During the follow-up period, we contacted them annually to collect information regarding the outcome of interest, such as death, major cardiovascular events, cardiac intervention, stroke, hospital admission, starting a new prescription, or being diagnosed with a chronic disease. The participants were informed about calling or filling out the form to report the incidence of any of the targeted outcomes as soon as possible. Two hospitals in Bushehr (ShohadayeKhalij-e-Fars and Salman-e-Farsi hospitals) were in charge of checking the Hospital Information System (HIS) and reporting all admissions of the participants. Then, a physician checked the medical records and contacted the research institution to register the specific information if they reported any expected outcomes. Moreover, our database was connected to the public health registry system database and social security services, which provided information from hospitals, clinics, established health care providers, cemeteries, and the department of social security services.
Ethics approval and consent to participate
The study was authorized by the ethical committee of Bushehr University of Medical Sciences (ref. no. b-91-14-2). It was carried out in accordance with the Helsinki Declaration and Iranian national norms for research ethics. Prior to research enrollment, all participants signed a written informed consent form. Participation was entirely independent, and anyone may withdraw their agreement at any moment with no repercussions. The information gathered is saved in a re-identifiable format using a national id code.
Anthropometric measurement, blood collection, and biochemical parameters
Trained expert measured the anthropometric sizes [height, weight, waist circumference, hip circumference, BMI, Waist to Hip Ratio (WHR)] according to the previously reported standard protocol [1,2]. They also collected fasting blood and sent the samples to the Persian Gulf Tropical Medicine Research Centre lab. The method of measurement is similar to previous studies [1,2].
Evaluation criteria
The definition of metabolic syndrome was based on the revised NCEP ATP III [30], definition and Iranian criteria for obesity [31,32], which is confirmed by having three or more of the following criteria;
(1) abdominal obesity (WC>102 cm in men or >88 cm in women).
(2) fasting TG level over 150 mg/dl.
(3) low HDL-cholesterol (<40 mg/dl in men or <50 mg/dl in women).
(4) increased blood pressure (blood pressure over 130/85 mmHg, or taking of anti-hypertensive medications) and
(5) impaired fasting glucose (fasting glucose ≥100 mg/dl or use of insulin or hypoglycemic medication).
VAI, a sex-specific index, was calculated by using the following formula [13]:
LAP is a sex-specific index as well, and the calculation formula is [18]:
Male LAP = [WC (cm) − 65] × TG concentration (mmol/l).
Female LAP = [WC (cm) − 58] × TG concentration (mmol/l).
We considered age >65 years, BMI ≥27 kg/m2, WC>94.5 cm for men (32) and >90 cm for women (31), WHR≥0.89 as a population at risk of developing MetS. The Body Adiposity Index (BAI) was also calculated, which is based on the hip circumference and stature data (BAI = hip circumference (cm)/height (m) 1.5-18). Different levels of VAI and LAP were tested to find a cutoff value that can predict the incidence of MetS. The odds ratio of VAI≥1. 88 and LAP≥37.10 for MetS occurrence were higher than other components.
Statistical analysis
Data analysis was done using Stata MP (version 15) software. The quantitative and qualitative data were presented as mean ± SD and percent (%), respectively. Logistic regression analysis measured the odds ratio (OR) of MetS for the defined risk factors and components of MetS (Table 2). Also, the incidence ratio of MetS based on the presence of defined risk factors and components was assessed (Table 3). Finally, the sensitivity, specificity, PPV, NPP, and AUC of defined risk factors for developing MetS were calculated (Table 4). P-value < 0.05 was defined as significant.
Results
The baseline characteristics of participants in two phases of cohort are shown in Table 1. Among those 3000 individuals who enrolled in phase I, 2,426 enrollees (80.87% response rate) participated in phase II and completed follow-up. At the baseline of the study, the mean age ± SD was 67.85 ± 7.10, and 1545 (51.5%) of the participants were women. In phase I, 1694 (56.47%) participants were diagnosed with MetS, and prevalence among the women and men were 1018 (65.89%) and 676 (46.46%), respectively. In phase 2, MetS was detected in 862 (68.58%) women and 355 (30.45) men, and overall, in 1,217 (50.23%) participants.
Table 1: Characteristics of Bushehr Elderly Cohort Study Population during phases I and II.
| Variables | Phase I (N = 3,000) | Phase II (N = 2,426) | |
|---|---|---|---|
| Age (years) | 67.85 ± 7.10 | 69.34 ± 6.39 | |
| Sex (Female, %) | 51.50% | 52 % | |
| Marital status, (%) | Single | 0.83 % | 0.83 % |
| Married | 74.93% | 74.93 % | |
| Divorced | 0.87% | 0.87% | |
| Widow | 23.37% | 23.37% | |
| current smoking (Cigarette, Hookah, and pipe), (%) | 19.88% | 18.40 % | |
| lifetime smoking (Cigarette, Hookah, pipe), (%) | 43.19% | 45.20% | |
| BMI (kg/m2) | 27.09 ± 4.99 | 27.51 ± 4.99 | |
| WC (cm) | 89.56 ± 17.97 | 98.71 ± 12.01 | |
| WHR | 0.89 ± 0.11 | 0.96 ± 0.08 | |
| TC (mg/dl) | TC (mg/dl) | 182. 18 ± 44.14 | |
| LDL (mg/dl) | 122.89 ± 39.70 | 109.43 ± 37.70 | |
| HDL (mg/dl) | 46.72 ± 13.20 | 45.94 ± 11.21 | |
| TG (mg/dl) | 135.92 ± 70.43 | 144.20 ± 75.37 | |
| FBS (mg/dl) | 110.03 ± 48.35 | 106.22± 42.58 | |
| SBP (mmHg) | 134.70 ± 19.54 | 139.65 ± 19.32 | |
| DBP (mmHg) | 76.38 ± 8.33 | 81.55 ± 8.67 | |
| MetS, N (%) | 1694 (56.47%) | 1217 (50.23%) | |
| LAP | 49.25 ± 34.01 | 59.14±39.72 | |
| VAI | 2.43 ± 1.93 | 2.51± 1.96 | |
| Continuous and categorical variables were present as mean ± SD and percent (%), respectively. | |||
The odds ratio of VAI≥1. 88 and LAP≥37.10 for MetS occurrence were higher than other components of MetS (OR (95%CI): 12.95 (10. 87, 15.44), p<0.001 for VAI>1.88 and 11.91 (10.02, 14.16), p<0.001 for LAP≥37.10), except for triglyceride (Table 2). Incidence rate ratios of MetS were also higher for VAI>1.88 and LAP≥37.10 compared to the other considered risk factors (Table 3).
Table 2: Odds ratios of metabolic syndrome according to the related risk factors.
| OR (95% CI), for men | p value | OR (95% CI), for women | p value | OR (95% CI), for total population | p value | |
|---|---|---|---|---|---|---|
| Age > 65 years | 0.78 (0.64, 0.96) | 0.023 | 0. 98 (0.79, 1.21) | 0.898 | 0.86 (0.74, 0.99) | 0.046 |
| BMI (kg/m2) ≥ 27 kg/m2 | 4.28 (3.42, 5.37) | <0.001 | 2.64 (2.13, 3. 28) | <0.001 | 3.68 (3.16, 4.29) | <0.001 |
| WC ≥ 94.5 cm in men or ≥90 cm in women | 5.15 (4.08, 6.51) | <0.001 | 3.06 (2.46, 3.82) | <0.001 | 4.21 (3.60, 4.93) | <0.001 |
| WHR ≥ 0.89 | 2.80 (2.17, 3.62) | <0.001 | 1.19 (0.96, 1.48) | 0.094 | 1.27 (1.10, 1.46) | 0.001 |
| TC (mg/dl) ≥ 200 mg/dl | 0.78 (0.59, 1.01) | 0.068 | 0.51 (0.35, 0.75) | <0.001 | 0.76 (0.62, 0.94) | 0.011 |
| TG (mg/dl) ≥ 150 mg/dl | 17.30 (12.81, 23.35) | <0.001 | 19. 08 (12.94, 28.15) | <0.001 | 16.77 (13.30, 21.14) | <0.001 |
| LDL (mg/dl) ≥ 130 mg/dl | 0.99 (0. 80, 1.22) | 0.939 | 0. 82 (0.66, 1.01) | 0.072 | 0.98 (0.85, 1.13) | 0.832 |
| HDL (mg/dl) < 40 mg/dl in men or < 50 mg/ dl in women | 9.32 (7.27, 11.94) | <0.001 | 11.02 (8.54, 14.22) | <0.001 | 10.73 (8.99, 12.80) | <0.001 |
| FBS (mg/dl) ≥ 100 mg/dl or use DM medication | 5.93 (4. 68, 7.50) | <0.001 | 8.81 (6.62, 11.73) | <0.001 | 6.66 (5.59, 7.93) | <0.001 |
| SBP ≥ 130 mmHg | 2.72 (2.20, 3.37) | <0.001 | 3.00 (2.41, 3.72) | <0.001 | 2.84 (2.44, 3.29) | <0.001 |
| DBP ≥ 85 mmHg | 1.79 (1.31, 2.44) | <0.001 | 1.58 (1.12, 2.22) | 0.008 | 1.65 (1.31, 2.07) | <0.001 |
| VAI ≥ 1. 88 | 13.94 (10.79, 18.01) | <0.001 | 10.57 (8.27, 13.51) | <0.001 | 12.95 (10. 87, 15.44) | <0.001 |
| LAP ≥ 37.10 | 14.96 (11.58, 19.31) | <0.001 | 8.65 (6.80, 11.00) | <0.001 | 11.91 (10.02, 14.16) | <0.001 |
Table 3: Incidence rate ratios of metabolic syndrome according to the related risk factors.
| Incidence ratio (95% CI) for men | p value | Incidence ratio (95% CI) for women | p value | Incidence ratio (95% CI) for total population | p value | |
|---|---|---|---|---|---|---|
| Age > 65 years | 0.86 (0.73, 1.01) | 0.069 | 0.86 (0.75, 0.99) | 0.045 | 0.86 (0.77, 0.96) | 0.008 |
| BMI (kg/m2) ≥ 27 kg/m2 | 1.48 (1.26, 1.73) | <0.001 | 1.21 (1.05, 1.39) | 0.006 | 1.36 (1.23, 1.51) | <0.001 |
| WC ≥ 94.5 cm in men or ≥90 cm in women | 1.94 (1.62, 2.34) | <0.001 | 1.62 (1.33, 2.00) | <0.001 | 1.85 (1.62, 2.12) | <0.001 |
| WHR ≥ 0.89 | 6.03 (3.25, 12.63) | <0.001 | 1.45 (1.20, 1.76) | <0.001 | 1.70 (1.43, 2.04) | <0.001 |
| TC (mg/dl) ≥ 200 mg/dl | 0.95 (0. 78, 1.14) | 0.2950 | 0.85 (0.74, 0.97) | 0.0221 | 0.92 (0.82, 1.02) | 0.140 |
| TG (mg/dl) ≥ 150 mg/dl | 2.39 (2.04, 2.80) | <0.001 | 1.70 (1.49, 1.94) | <0.001 | 1.99 (1.79, 2.20) | <0.001 |
| LDL (mg/dl) ≥ 130 mg/dl | 0.90 (0.75, 1.09) | 0.3172 | 0.86 (0.74, 0.99) | 0.0401 | 0.90 (0.80, 1.01) | 0.077 |
| HDL (mg/dl) < 40 mg/dl in men or < 50 mg/ dl in women | 2.82 (2.15, 3.74) | <0.001 | 2.31 (1.98, 2.70) | <0.001 | 2.23 (1.96, 2.55) | <0.001 |
| Fasting glucose (mg/dl) ≥ 100 mg/dl or use DM medication | 0.67 (0.01, 3.39) | 0.7089 | 0.60 (0.01, 3.38) | 0.7018 | 0.66 (0.01, 3.73) | 0.7847 |
| SBP ≥ 130 mmHg | 1.42 (1.19, 1.71) | <0.001 | 1.36 (1.17, 1.58) | <0.001 | 1.38 (1.23, 1.56) | <0.001 |
| DBP ≥ 85 mmHg | 1.12 (0.96, 1.32) | 0.1353 | 1.11 (0.96, 1.28) | 0.1363 | 1.09 (0.98, 1.21) | 0.0777 |
| VAI ≥ 1. 88 | 2.79 (2.36, 3.30) | <0.001 | 2.29 (1.92, 2.73) | <0.001 | 2.55 (2.26, 2.87) | <0.001 |
| LAP ≥ 37.10 | 2.61 (2.17, 3.17) | <0.001 | 2.16 (1.76, 2.68) | <0.001 | 2.45 (2.13, 2.82) | <0.001 |
Table 4: The Curve Analysis, Sensitivity, Specificity, Positive and Negative Predictive Values of Parameters for Metabolic Syndrome.
| AUC (95% CI) | p value | Sensitivity % | Specificity % | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|---|---|
| Age > 65 years | 0.43 (0.40 to 0.46) | 1.000 | 0.86 | 0.11 | 0.54 | 0.40 |
| BMI (kg/m2) ≥ 27 kg/m2 | 0.55 (0.51 to 0.58) | 0.001 | 0.99 | 0.00 | 0.73 | 0.00 |
| WC ≥ 94.5 cm in men or ≥90 cm in women | 0.56 (0.52 to0.59) | <0.001 | 0.92 | 0.08 | 0.72 | 0.30 |
| WHR ≥ 0.89 | 0.57 (0.54 to 0.60) | <0.001 | 0.99 | 0.00 | 0.62 | 0.00 |
| TC (mg/dl) ≥ 200 mg/dl | 0.56 (0.53 to 0.59) | <0.001 | 0.98 | 0.02 | 0.58 | 0.52 |
| TG (mg/dl) ≥ 150 mg/dl | 0.57 (0.54to 0.60) | <0.001 | 0.99 | 0.00 | 0.63 | 0.36 |
| LDL (mg/dl) ≥ 130 mg/dl | 0.55 (0.52 to 0.59) | <0.001 | 0.96 | 0.06 | 0.56 | 0.50 |
| HDL (mg/dl) < 40 mg/dl in men or < 50 mg/ dl in women | 0.45 (0.42 to 0.48) | 0.996 | 0.99 | 0.00 | 0.40 | 0.75 |
| FBS (mg/dl) ≥ 100 mg/dl or use DM medication | 0.56 (0.51 to 0.60) | 0.001 | 0.96 | 0.04 | 0.81 | 0.23 |
| SBP ≥ 130 mmHg | 0.52 (0.50 to 0.55) | 0.022 | 0.94 | 0.07 | 0.67 | 0.41 |
| DBP ≥ 85 mmHg | 0.49 (0.43 to 0.54) | 0.588 | 0.82 | 0.17 | 0.67 | 0.33 |
| VAI ≥ 1. 88 | 0.86 (0.84 to 0.87) | <0.001 | 0.76 | 0.80 | 0.83 | 0.72 |
| LAP ≥ 37.10 | 0.73 (0.71 to 0.75) | <0.001 | 0.74 | 0.62 | 0.67 | 0.69 |
Moreover, the obesity indicators, VAI and LAP, seem to have better specificity and almost similar sensitivity compared to the other components for detecting MetS. They have higher AUC (area under the curve) values for predicting MetS (Table 4). It generally seems that VAI and LAP are acceptable predictors for MetS.
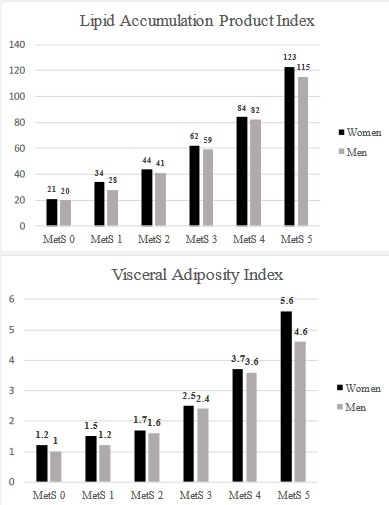
As shown in figure 1, we observed a proportional increment in obesity indicators, VAI and LAP, as the number of risk factors for MetS raised (Figure 1).
Supplementary tables of metabolic syndrome diagnosis based on Visceral Adiposity Index (VAI) and Lipid Accumulation Product (LAP).
Discussion
In this cohort study on the Iranian elderly population, the obesity indicators, VAI and LAP, were significantly associated with MetS, and these indicators predict MetS better than other MetS risk factors.
VAI has a positive correlation with hypertension [33], diabetes [11], Non-Alcoholic Fatty Liver Disease (NAFLD) [34,35], Non-Alcoholic Steatohepatitis (NASH) [35], cardiovascular events [36], and diabetes complications, such as cardiovascular disease and DM-related chronic kidney disease [37]. It also positively correlates with subclinical atherosclerosis [38] and silent brain infarct [39]. LAP is an obesity indicator as well. It has a positive correlation with cardiometabolic risk [40], NAFLD [41], diabetes [42], impaired fasting glucose [43], hypertension [44], late-onset hypogonadism in men [45], and cognitive impairment [46]. The growing evidence of positive correlation and predictive role of VAI and LAP for non-communicable diseases in literature underscores the importance of including these adiposity indices in clinical practice.
Some previous studies in line with the results of our study show that the VAI and LAP can be good predictors for MetS. In a cross-sectional study with 152 postmenopausal women, VAI was the best predictor index for MetS from all adiposity indices assessed. This study includes only women and relatively low sample size is mentioned as one of its limitations [16]. A recent meta-analysis study has introduced VAI as an accurate, low-cost and accessible method for MetS screening in adults. However, it is recommended that more studies should be conducted to evaluate clinical applicability, achieve the optimal cut-off, and identify the population that will benefit the most [17]. A crosssectional study with 411 subjects >60 years, indicated that LAP is a better cardiovascular risk predictor in elderly populations than other anthropometric measures [28]. In this study, insulin resistance is considered as the main cause of cardiometabolic diseases. The cut-off for LAP to determine the risk of insulin resistance is slightly higher than the cut-off determined in our study, which seems reasonable considering that our outcome is MetS, as a set of disorders. Consistently another review has also identified the LAP as the best method in identifying MetS among the elderly people [29].
An increase in the prevalence of obesity was concurrent with an increased incidence of diabetes and cardiovascular disease [47,48]. Previous studies have shown that central/visceral obesity is associated with MetS [49,50]. Visceral fat is highly metabolically dynamic tissue and is constantly releasing Free Fatty Acids (FFA) and pro-inflammatory cytokines into the blood stream. Therefore visceral fat induces hyperinsulinemia, systemic inflammation, dyslipidemia, and atherosclerosis as various features of the MetS [51].
There are different techniques to assess body composition, such as densitometry, air displacement plethysmography, electrical impedance analysis, DXA, CT, and MRI. DXA and MRI seem to be more precise but are not applicable for the whole population because of expense and time [10]. Applying WHR and WC could potentially improve the predictive power of obesity in estimating cardiovascular risk. It adds significant information beyond BMI, reflecting the importance of intra-abdominal fat accumulation [4,50,52-54]. However, after using components of MetS in a simple mathematical equation and generating adiposity indicators, VAI, and LAP, we could improve the sensitivity and specificity of detecting and even predicting MetS. Improving the diagnosis of MetS in elderly high-risk populations is crucial to allocate the medical resources precisely. With the improvement of diagnosis and early approach, we will be able to alleviate the economic burden and severe complications of MetS, such as CVDs and diabetes in elderly patients.
However, the current study faces some limitations including loss of enrollees during the second phase, and failure to assess participants' food and drug intake as important confounders of the study.
Conclusion
In conclusion, VAI and LAP are relatively strong indicators of obesity, especially visceral obesity, with strong predictability for MetS in elderly. They are simple to measure and applicable for clinical practice.
Abbreviations: ABSI: A Body Shape Index; AUC: Area Under The Curve; BAI: Body Adiposity Index; BEH: Bushehr Elderly Health; BMI: Body Mass Index; CT: Computed Tomography; CVA: Cerebrovascular Disease; CVD: Cardiovascular Disease; DBP: Diastolic Blood Pressure; DXA: Dual-Energy X-Ray Absorptiometry; HC: Hip Circumference; HDL: High-Density Lipoprotein; LAP: Lipid Accumulation Product; Mets: Metabolic Syndrome; MRI: Magnetic Resonance Imaging; NAFLD: Non-Alcoholic Fatty Liver Disease; NASH: Non-Alcoholic Steatohepatitis; NPV: Negative Predictive Value; PPV: Positive Predictive Value; SBP: Systolic Blood Pressure; SD: Standard Deviation; T2DM: Type 2 Diabetes; TG: Serum Triglycerides; TC: Total Cholesterol; VAD: Visceral Adipose Dysfunction; VAI: Visceral Adiposity Index; VAT: Visceral Adipose Tissue; WC: Waist Circumference; WHR: Waist To Hip Ratio.
Declarations
Acknowledgments: The authors take this opportunity to express their appreciation of Artin Asadipooya at the University of Kentucky for editing this article. They also demonstrate their gratitude to the staff of Shohadaye Khalij-e-Fars hospital and Persian Gulf Tropical Medicine Research Center at the Bushehr University of Medical Sciences for their kind collaboration in collecting data.
Funding: Funding for BEH Program was jointly provided by the Persian Gulf Biomedical Sciences Research Institute affiliated with Bushehr (Port) University of Medical Sciences (BPUMS) and the Endocrinology and Metabolism Research Institute, affiliated with Tehran University of Medical Sciences (TUMS). Researchers from both research centers have contributed to the design and conduct of this research project.
Ethics approval and consent to participate: The study was approved by the Ethics Committee of Bushehr University of Medical Sciences (ref. No. B-91–14-2). This study was conducted in agreement with the Declaration of Helsinki and Iranian national guidelines for ethics in research. Written informed consent was obtained from all participants prior to study enrolment. Participation was voluntary, and each participant could withdraw consent at any time without any consequence. Data collected were stored in a re-identifiable form by national ID code.
Competing interests: The authors declare no conflict of interest
Consent for publication: Not applicable.
Author contribution
MM: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization.
KA: Writing - original draft, Writing - review& editing.
IN: principal investigator, Resources, Data curation, review & editing.
MG Data curation, review & editing.
AO: Resources, review& editing.
BL: review & editing.
AF: Conceptualization, review& editing.
AHD: Resources, Writing - review & editing.
FM: review & editing.
MK: Conceptualization, Resources, Writing - review& editing, Supervision
References
- Ostovar A, Nabipour I, Larijani B, Heshmat R, Darabi H, et al. Bushehr Elderly Health (BEH) Programme, phase I (cardiovascular system). BMJ open. 2015; 5: e009597.
- Shafiee G, Ostovar A, Heshmat R, Darabi H, Sharifi F, et al. Bushehr Elderly Health (BEH) programme: study protocol and design of musculoskeletal system and cognitive function (stage II). BMJ open. 2017; 7: e013606.
- Engin A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Advances in experimental medicine and biology. 2017; 960: 1-17.
- Després JP. Body fat distribution and risk of cardiovascular disease: An update. Circulation. 2012; 126: 1301-1313.
- Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: An endocrine organ. Arch Med Sci. 2013; 9: 191-200.
- Mittal B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J Med Res. 2019; 149: 571-573.
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. The Journal of clinical endocrinology and metabolism. 2004; 89: 2548-2556.
- Ruiz-Castell M, Samouda H, Bocquet V, Fagherazzi G, Stranges S, et al. Estimated visceral adiposity is associated with risk of cardiometabolic conditions in a population based study. Scientific Reports. 2021; 11: 9121.
- Rheaume C, Leblanc ME, Poirier P. Adiposity assessment: Explaining the association between obesity, hypertension and stroke. Expert review of cardiovascular therapy. 2011; 9: 1557-1564.
- Borga M, West J, Bell JD, Harvey NC, Romu T, et al. Advanced body composition assessment: from body mass index to body composition profiling. Journal of investigative medicine : The official publication of the American Federation for Clinical Research. 2018; 66: 1-9.
- Nusrianto R, Tahapary DL, Soewondo P. Visceral adiposity index as a predictor for type 2 diabetes mellitus in Asian population: A systematic review. Diabetes & metabolic syndrome. 2019; 13: 1231-1235.
- Zheng SH, Li XL. Visceral adiposity index as a predictor of clinical severity and therapeutic outcome of PCOS. Gynecological endocrinology : The official journal of the International Society of Gynecological Endocrinology. 2016; 32: 177-183.
- Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes care. 2010; 33: 920-922.
- Biswas E, Choudhury AK, Amin MR, Khalequzzaman M, Chowdhury S, et al. Visceral Adiposity Index Score is the Better Predictor of Clinical and Coronary Angiographic Severity Assessment than Other Adiposity Indices in Patients with Acute Coronary Syndrome. Mymensingh medical journal : MMJ. 2019; 28: 382-388.
- Randrianarisoa E, Lehn-Stefan A, Hieronimus A, Rietig R, Fritsche A, et al. Visceral Adiposity Index as an Independent Marker of Subclinical Atherosclerosis in Individuals Prone to Diabetes Mellitus. Journal of atherosclerosis and thrombosis. 2019; 26: 821-834.
- Correia ES, Godinho-Mota JCM, Schincaglia RM, Martins KA, Martins JS, et al. Metabolic Syndrome in postmenopausal women: prevalence, sensibility, and specificity of adiposity indices. Clinical Nutrition Open Science. 2022; 41: 106-114.
- Bijari M, Jangjoo S, Emami N, Raji S, Mottaghi M, et al. The Accuracy of Visceral Adiposity Index for the Screening of Metabolic Syndrome: A Systematic Review and Meta-Analysis. International Journal of Endocrinology. 2021; 2021: 6684627.
- Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC cardiovascular disorders. 2005; 5: 26.
- Nusrianto R, Ayundini G, Kristanti M, Astrella C, Amalina N, et al. Visceral adiposity index and lipid accumulation product as a predictor of type 2 diabetes mellitus: The Bogor cohort study of non-communicable diseases risk factors. Diabetes research and clinical practice. 2019; 155: 107798.
- Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes care. 2006; 29: 151-3.
- Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC gastroenterology. 2010; 10: 98.
- Cheng YL, Wang YJ, Lan KH, Huo TI, Huang YH, et al. Fatty Liver Index and Lipid Accumulation Product Can Predict Metabolic Syndrome in Subjects without Fatty Liver Disease. Gastroenterology research and practice. 2017; 2017: 9279836.
- Ioachimescu AG, Brennan DM, Hoar BM, Hoogwerf BJ. The lipid accumulation product and all-cause mortality in patients at high cardiovascular risk: a PreCIS database study. Obesity (Silver Spring, Md). 2010; 18: 1836-1844.
- Bozorgmanesh M, Hadaegh F, Azizi F. Predictive performances of lipid accumulation product vs. adiposity measures for cardiovascular diseases and all-cause mortality, 8.6-year follow-up: Tehran lipid and glucose study. Lipids in health and disease. 2010; 9: 100.
- Gao X, Wang G, Wang A, Xu T, Tong W, et al. Comparison of lipid accumulation product with body mass index as an indicator of hypertension risk among Mongolians in China. Obesity research & clinical practice. 2013; 7: e308-14.
- Dai D, Chang Y, Chen Y, Chen S, Yu S, et al. Visceral Adiposity Index and Lipid Accumulation Product Index: Two Alternate Body Indices to Identify Chronic Kidney Disease among the Rural Population in Northeast China. International journal of environmental research and public health. 2016; 13.
- Maturana MA, Moreira RM, Spritzer PM. Lipid accumulation product (LAP) is related to androgenicity and cardiovascular risk factors in postmenopausal women. Maturitas. 2011; 70: 395-399.
- Nunes SH, Nogueira Saad MA, da Cruz Filho RA, Jorge AJL, Santos MMSd, Martins WdA, et al. Is lipid accumulation product a better cardiovascular risk predictor in elderly individuals than anthropometric measures? Revista Portuguesa de Cardiologia (English Edition). 2021; 40: 539-544.
- Oliveira CCd, Costa EDd, Roriz AKC, Ramos LB, Gomes M. Predictors of Metabolic Syndrome in the elderly: A review. International Journal of Cardiovascular Sciences. 2017; 30: 343-353.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112: 2735-2752.
- Azizi F, Khalili D, Aghajani H, Esteghamati A, Hosseinpanah F, et al. Appropriate waist circumference cut-off points among Iranian adults: the first report of the Iranian National Committee of Obesity. Archives of Iranian medicine. 2010; 13: 243-244.
- Gharipour M, Sadeghi M, Dianatkhah M, Bidmeshgi S, Ahmadi A, et al. The cut-off values of anthropometric indices for identifying subjects at risk for metabolic syndrome in Iranian elderly men. Journal of obesity. 2014; 2014: 907149.
- Ding Y, Gu D, Zhang Y, Han W, Liu H, et al. Significantly increased visceral adiposity index in prehypertension. PloS one. 2015; 10: e0123414.
- Vassilatou E, Lafoyianni S, Vassiliadi DA, Ioannidis D, Paschou SA, et al. Visceral adiposity index for the diagnosis of nonalcoholic fatty liver disease in premenopausal women with and without polycystic ovary syndrome. Maturitas. 2018; 116: 1-7.
- Vural Keskinler M, Mutlu HH, Sirin A, Erkalma Senates B, Colak Y, et al. Visceral Adiposity Index As a Practical Tool in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Metabolic syndrome and related disorders. 2020.
- Amato MC, Giordano C, Pitrone M, Galluzzo A. Cut-off points of the Visceral Adiposity Index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids in health and disease. 2011; 10: 183.
- Wan H, Wang Y, Xiang Q, Fang S, Chen Y, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovascular diabetology. 2020; 19: 118.
- Park HJ, Kim J, Park SE, Park CY, Lee WY, et al. Increased risk of subclinical atherosclerosis associated with high visceral adiposity index in apparently healthy Korean adults: the Kangbuk Samsung Health Study. Annals of medicine. 2016; 48: 410-416.
- Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM, et al. Visceral adiposity index is associated with silent brain infarct in a healthy population. Sci Rep. 2020; 10: 17271.
- Demirbas N, Kutlu R. Importance of Measured Body Fat, Visceral Adiposity Index, and Lipid Accumulation Product Index in Predicting Cardiometabolic Risk Factors. Metabolic syndrome and related disorders. 2020.
- Silveira EA, Kliemann N, Noll M, Sarrafzadegan N, de Oliveira C. Visceral obesity and incident cancer and cardiovascular disease: An integrative review of the epidemiological evidence. Obesity reviews : An official journal of the International Association for the Study of Obesity. 2020.
- Fu W, Wang C, Zou L, Jiang H, Miller M, et al. Association of adiposity with diabetes: A national research among Chinese adults. Diabetes/metabolism research and reviews. 2020: e3380.
- Song J, Chen X, Jiang Y, Mi J, Zhang Y, et al. Association and Interaction Analysis of Lipid Accumulation Product with Impaired Fasting Glucose Risk: A Cross-Sectional Survey. Journal of diabetes research. 2019; 2019: 9014698.
- Huang J, Bao X, Xie Y, Zhang X, Peng X, et al. Interaction of lipid accumulation product and family history of hypertension on hypertension risk: A cross-sectional study in the Southern Chinese population. BMJ open. 2019; 9: e029253.
- Sun K, Wang C, Lao G, Lin D, Huang C, et al. Lipid accumulation product and late-onset hypogonadism in middle-aged and elderly men: results from a cross-sectional study in China. BMJ open. 2020; 10: e033991.
- Yu ZW, Li X, Wang Y, Fu YH, Gao XY. Association Between Lipid Accumulation Product and Mild Cognitive Impairment in Patients with Type 2 Diabetes. Journal of Alzheimer’s disease : JAD. 2020; 77: 367-374.
- Trends in cardiometabolic risk factors in the Americas between 1980 and 2014: A pooled analysis of population-based surveys. The Lancet Global health. 2020; 8: e123-e33.
- Lanas F, Seron P. Diverging trends in obesity, diabetes, and raised blood pressure in the Americas. The Lancet Global health. 2020; 8: e18-e9.
- Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. The lancet Diabetes & endocrinology. 2019; 7: 313-324.
- Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. The lancet Diabetes & endocrinology. 2020; 8: 616-627.
- Chait A, Den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Frontiers in cardiovascular medicine. 2020; 7: 22.
- Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, et al. Causal Associations of Adiposity and Body Fat Distribution With Coronary Heart Disease, Stroke Subtypes, and Type 2 Diabetes Mellitus: A Mendelian Randomization Analysis. Circulation. 2017; 135: 2373-2388.
- Cameron AJ, Magliano DJ, Söderberg S. A systematic review of the impact of including both waist and hip circumference in risk models for cardiovascular diseases, diabetes and mortality. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013; 14: 86-94.
- Nalini M, Sharafkhah M, Poustchi H, Sepanlou SG, Pourshams A, et al. Comparing Anthropometric Indicators of Visceral and General Adiposity as Determinants of Overall and Cardiovascular Mortality. Archives of Iranian medicine. 2019; 22: 301-309.