Research Article
Volume 2, Issue 6
Hydrogen Gas Device has Therapeutic Effect on Skin Damage and Alleviates Dermatitis in Mice
Vladimir I Klichko1; Daniel H Crow2; Svetlana N Radyuk1*
1Department of Biological Sciences, Southern Methodist University, 6501 Airline Rd., Dallas, Texas 75275, USA.
2Hyderm Innovations, Inc., 3526 Arrowhead Dr., Dallas, Texas 75204, USA.
Corresponding Author :
Svetlana N Radyuk
Tel: +1-214-768-2892 & +1-214-768-3955
Email: snradyuk@smu.edu
Received : May 27, 2023 Accepted : Jun 16, 2023 Published : Jun 23, 2023 Archived : www.meddiscoveries.org
Citation: Klichko VI, Crow DH, Radyuk SN. Hydrogen Gas Device has Therapeutic Effect on Skin Damage and Alleviates Dermatitis in Mice. Med Discoveries. 2023; 2(6): 1048.
Copyright: © 2023 Radyuk SN. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Molecular hydrogen (H2) emerged a decade ago as a potent therapeutic and is attracting more and more attention. The beneficial effects of H2 are ascribed to its antioxidant and anti-inflammatory function, as well as its ability to induce defense responses and confer protective effects. Remarkably, H2 has no known adverse effects on cell function.
Substantial H2 is produced by the intestinal microflora under physiological conditions [1]. However, concentrations of endogenously produced H2 is not sufficient to treat disease or restore normal physiology. Notable effects are observed only when exogenous H2 is applied. H2 is administered via inhalation, oral intake, or injection of H2 saturated solution [2,3]. It can also be applied transdermally by applying H2 saturated water or mixing chemicals together that react to form H2 [2,3]. All these approaches achieve a transient increase in the concentration of H2, along with beneficial effects on cell function [4-8]. Although administration of H2 by inhalation or ingestion is the predominant method, application of H2 transdermally provides targeted delivery to specific tissue, allowing for a local effect with a powerful H2 concentration gradient.
When applied topically, H2 not only acts on underlying skin and mucosal tissue, but is absorbed through the epidermis and delivered directly to damaged muscle and nerves. The aim of this study is to test a method for delivering H2 to skin and subcutaneous tissue and demonstrate its effectiveness in treating skin lesions. For that purpose, we developed an H2-producing device to treat a mouse model of oxazolone-induced dermatitis. Oxazolone is a chemical known to cause skin sensitization, allergy-like symptoms, and oxidative damage. More specifically, oxazolone is capable of causing skin inflammatory lesions similar to those in atopic dermatitis and contact dermatitis [9]. Since H2 is known for its antioxidant and anti-inflammatory activity, we were able to demonstrate the efficacy of the developed method on dermatitis-like skin lesions.
Materials and methods
Animals
All experimental procedures and a humane euthanasia were performed according to the protocol approved by SMU Institutional Animal Care and Use Committee. Eight week old male mice strain SKH-1 (hairless) was purchased from the Charles River Laboratories (Wilmington, MA, USA). Mice were maintained at ambient temperature 25 ± 2ºC and 40-60% humidity under a 12 h light-dark cycle.
Induction of a dermatitis-like skin lesions
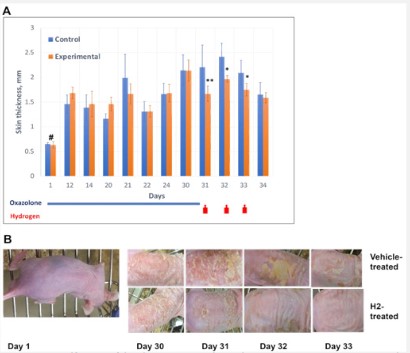
Mice were exposed to 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone, Sigma-Aldrich), a chemical that causes atopic dermatitis-like skin lesions [10]. Oxazolone was repeatedly applied topically over a long period according to schemes [11-13]. Briefly, animals (n=10) were sensitized by applying 0.5% oxazolone dissolved in a mixture of acetone and olive oil (3:1). 200 μL of oxazolone solution was applied to an area of ~1 x 3 cm of dorsal skin three times per week for two weeks. Allergy symptoms developed based on the appearance of thick, scaly, red skin where oxazolone was applied (Figure S1) and animals were then randomly divided in two groups, experimental (n=5) and control (n=5). Sensitization with oxazolone continued for another two weeks followed by the therapeutic device treatment (Figure 1). During this period, both groups were treated with 200 μL of 0.2% oxazolone to dorsal skin three times per week.
The degree of skin lesions was evaluated based on visual assessment of skin redness and scaling (Figure S1). The intensity of skin redness was assessed based on scores none (0), mild (1), moderate (2), severe (3), and extremely severe (4) according to intensity of color (Figure S1) [14]. Degree of skin lesions were also assessed by measuring skin thickness in the middle of the back of mice using QUICKmini micrometer (Mitutoyo Corp., Japan).
Design of the hydrogen-generating device
H2 was delivered to the skin using a device made in the laboratory. The device consisted of a bag made of a KT-like tape (from a hydrophobic waterproof woven material of CVS brand) and a Mylar® film (DuPont) soldered together (Figure S2). Device contained aluminum foil inserts, calcium hydroxide, and filter paper. The chemical reaction to create H2 was initiated by adding a solution of sodium chloride (Figures S2-S3 for details). Bags were sealed and applied to damaged areas of the skin using adhesive plasters daily for 4 hours.
Device application
The experimental group was treated with a device that produced H2 gas from a chemical reaction. The device resembled a plastic bag taped to the skin. The control group was treated with an inert device that did not produce H2 because it lacked the catalyst, aluminum foil.
Skin lesions were assessed every day after start of therapeutic treatment. The degree of skin lesions, such as thickness, scaling, and redness, was determined as specified above. On the last day of the experiment (day 34), animals were sacrificed and H2 diffusion into tissue was assessed using a special electrode. Skin and serum samples were taken for determination of oxidative damage and markers of inflammation.
Hydrogen diffusion into the tissue
Diffusion of H2 into the skin was determined by using a hydrogen microsensor, a needle-like electrode (Unisense, Denmark) connected to a high-sensitivity picoammeter (Unisense Microsensor Monometer version 1.01), and corresponding software (SensorTraced SUITE v.3.1.5, Unisense, Denmark). Calibration and readings were conducted per the manual. Calibration curve was obtained using H2O saturated with H2 gas at room temperature. According to the literature, maximum concentrations of H2 gas dissolved in water at ambient temperature and normal atmosphere pressure is ~800 μM. H2 concentrations were calculated from the calibration curve using known levels of H2-saturated water.
Oxidative damage
Dorsal skin samples were taken from oxazolone-exposed areas and stored at -80°C. Skin homogenates for determination of malondialdehyde (MDA) as a biomarker of lipid peroxidation were prepared in RIPA buffer, followed by determination of protein concentration by the Lowry method (Biorad). Homogenates were standardized for protein concentration. MDA levels were determined using the TBARS assay kit (Cayman Chemicals) per manufacturer protocol.
Markers of inflammation
Blood samples drawn from sacrificed animals were evaluated for cytokine interleukin-1β (IL-1β) as a marker of pro-inflammatory response. Collected blood was allowed to coagulate, followed by aspiration of cell-free serum. Serum IL-1β levels were measured using the Mouse IL-1β ELISA Ready-SET-Go! kit (eBioscience Inc.) per manufacturer instructions.
Statistical analysis: Data was analyzed using Microsoft Excel for Mac and values were expressed as mean ± SD. Group means were analyzed and compared using one-way ANOVA followed by multiple comparison tests, detailed in Figure legends, using Prism version 9.0 software packages (GraphPad Software Inc.).
Results
Hydrogen-releasing patches reduced skin lesions caused by oxazolone
H2-producing packs were applied to the skin lesions of the experimental group. Inert packs were applied control group. H2 packs and oxazolone treatments were applied according to scheme (Figure 1A).
In both experimental and control, animals were monitored daily for changes to skin condition. Repeated oxazolone applications provoked dermatitis-like skin lesions entailing scaling, redness, and increased skin thickness (Figure 1). Lesions appeared after 3-5 days of oxazolone use. Desired degree of sustained skin damage, a “4” on our “0 to 4” scale (Figure S1), was achieved by day 30.
Introduction of the H2-producing device began once the mice established dermatitis-like symptoms from repeated oxazolone application (Figure 1). Effectiveness of the device was assessed visually and by measuring skin thickness as an indicator of inflammation/edema. Based on visual evaluation (Figure 1 and Figure S1), animals that received oxazolone without subsequent H2 device administration exhibited severe skin scaling and redness. Scaling and redness were significantly reduced in experimental group receiving the H2-releasing device (Figure 1B). Additionally, the experimental group experienced a slight but significant reduction in skin thickness.
After signs of recovery were established in the experimental group on day 4 post-treatment, mice were humanely euthanized, followed by tissue and blood sampling for analysis of cytokine levels and oxidative damage.
Effect of H2-relasing device on oxidative damage
Oxazolone exposure has been shown to cause oxidative stress and inflammation in many studies [15]. Oxidative stress and reactive oxygen species (ROS) production lead to the generation of lipid peroxides. Beyond being a useful biomarker of oxidative stress, lipid peroxides are cytotoxic and involve in a number of biological processes. An extensive body of literature points to lipid peroxides having a role in signaling mechanisms. In particular, reactions are mediated by lipid peroxidation products, such as malondialdehyde (MDA), the accumulation of which can lead to the development of various pathological processes (reviewed in) [16].
MDA is a product of free radical attacks on membrane lipoproteins and polyunsaturated fatty acids. MDA levels reflect a local degree of lipid peroxidation and cellular damage. Since oxazolone has been reported to increase levels of lipid peroxidation [17-19], oxidative damages caused by oxazolone were assessed by measuring MDA levels.
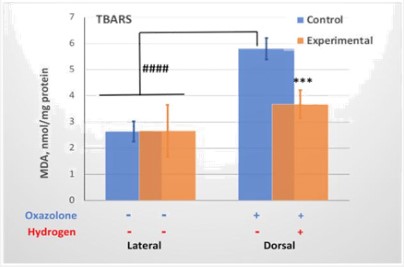
MDA levels were determined in skin samples taken from different sites (lateral and dorsal) using the TBARS assay. As shown in Figure 2, MDA levels were more than 2-fold higher in skin exposed to oxazolone, the dorsal skin. Application of the H2-releasing device significantly reduced MDA levels in the dorsal skin in the experimental group, but the lateral skin, which was not treated with oxazolone, did not experience changes in MDA levels. Ultimately, oxazolone caused local oxidative damage to the skin which was significantly reduced by H2 therapy, although no reduction to the levels of intact lateral skin was achieved.
Hydrogen-releasing device provided anti-inflammatory effects in mice
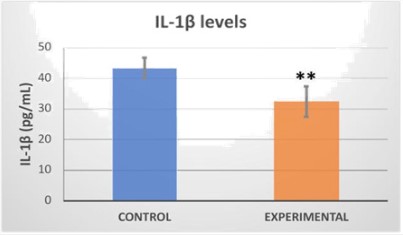
Levels of cytokines, key mediators of the inflammatory response, such as IL-1β, increase in response to oxazolone exposure [12,13,15]. Serum levels of cytokine IL-1β were deter- mined in order to evaluate the efficacy of the H2 device as a therapy for dermatitis-like lesions. We found that H2-treated mice showed significantly decreased serum concentrations of IL-1β when compared to control group (Figure 3).
A, Dorsal skin thickness of group treated with hydrogen and control group. Oxazolone was applied day 1 to 30. Hydrogen-releasing packs applied as shown in diagram by red arrows. Skin thickness values are mean ± SD (n=5). Significance was analyzed by 2-way ANOVA with Dunnett post-test. Significant difference (*p<0.05, **p<0.005) between inert vehicle-treated (Control) and hydrogen-treated (Experimental) animals is marked by asterisks. # marks a significant difference between time points. B, Representative skin images of mice with different treatments. Oxazolone has been applied through day 30. Hydrogen treatment started after day 30.
Control – mice not treated with hydrogen. Experimental – mice treated with H2. Values represent mean ± SD (n = 5). Data were analyzed by 2-way ANOVA and Tukey’s post-test. A significant difference (***p<0.0005) between vehicle-treated (Control) and hydrogen-treated (Experimental) animals marked by asterisk. ####p<0.0001 marks a significant difference between lateral (no exposure to oxazolone) and dorsal (oxazolone treated) skin samples.
Concentration of H2 under the skin
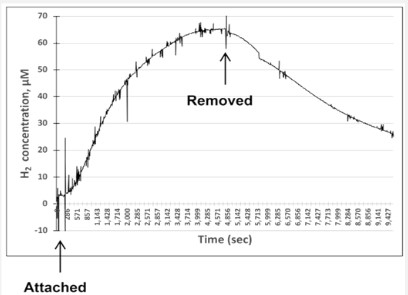
We tested three variants of devices containing different H2-producing compositions (Figure S4 and Figure 4). Shown in Figure 4 is the variant of the device used in this study. The obtained data shows that the device effectively delivers H2 to the targeted tissue, in this case the dorsal area. Device maintained a considerably high level of H2 for about an hour. Moreover, H2 did not return to baseline one hour after removing device.
Data obtained by using H2 electrode (Unisense) inserted under skin (Figure S4). Hydrogen-producing device was securely attached to area of skin with inserted electrode. Representative graph shows increase in H2 concentration for the variant of the H2 producing device used in study (Figure S4A). The bag is made of KT + Mylar and H2 is produced in a reaction detailed in Figure S2. Time points of device attachment and removal indicated by arrows. Maximum concentrations of H2 were 60-70 μM. After removal of the bag, H2 concentration slowly decreased.
Discussion
Here we tested a device that produces H2 gas by applying it topically to treat oxazolone-induced skin inflammation. For this purpose, we used the SKH-1 hairless mouse strain, ideal for studying skin conditions [20]. In particular, this model makes it possible to minimize the background inflammatory response caused by stress unrelated to the studied pathology, such as eliminating the need for epilation. Using this model, we were able to test the developed H2 gas-releasing device and demonstrate its therapeutic effect on mice with dermatitis-like skin lesions.
We chose oxazolone to induce the dermatitis-like lesions. This chemical acts as a hapten and capable of inducing immune system hypersensitivity reactions and allergy-like symptoms largely identical to those of atopic dermatitis and contact dermatitis in humans. These immunological abnormalities are associated with a pro-inflammatory response and increased production of ROS [10,12,13,15,21].
Using the approaches described in other studies [11,12,21], we achieved comparable oxazolone-provoked effects on the development of skin lesions, such as scaling and increased epidermal thickness (Figure 1), as well as an increase in tissue oxidative damage and pro-inflammatory cytokine levels (Figures 2,3).
Application of the H2-releasing device alleviated the severity of oxazolone-induced atopic dermatitis in SKH-1 mice, resulting in a significant reduction in skin damage. This was evident from reduced epidermal thickness and scaling (Figure 1). In a different study, similar effects in the treatment of skin lesions caused by UV radiation in hairless mice was achieved by bathing animals in electrolyzed reduced water, which is characterized by significant changes in redox potential [22]. Although H2 concentrations were not measured in that study, it is known that electrolysis of water produces H2. It is likely that the positive effects of such treatment were due to H2.
H2 is known for its antioxidant and anti-inflammatory activity [23,24]. The role of H2 in attenuating the hapten-induced inflammatory response is supported by finding that the increase in serum IL-1β was reduced by administration of the H2 releasing device. Moreover, our data showed that H2 had a systemic anti-inflammatory effect, as a decrease in IL-1β was detected in the blood (Figure 3). This suggests that application of the H2-releasing device protects against acute chemical injury by suppressing the skin’s inflammatory response.
The results of the H2-releasing device in this study are consistent with the anti-inflammatory effects using hydrogen-rich water in another studies. For example, hydrogen-rich water alleviated the severity of atopic dermatitis in NC/Nga mice by suppressing skin inflammation evident by decreased IL-1β [25,26]. Similar anti-inflammatory effects were observed in rats when hydrogen-rich water was used to reduce radiation-caused dermatitis and UVB injury [27,28].
Lower levels of cytokine IL-1β correspond to reduced levels of lipid peroxidation (Figures 2,3). Lipid peroxidation is a marker of inflammation and oxidative damage and plays an important role in dermatitis development. There is ample evidence that allergic and inflammatory skin diseases are mediated by oxidative stress (reviewed in Corsini) [29]. For example, in rats with skin injury caused by ischemia/reperfusion, both anti-oxidant and anti-inflammatory effects of hydrogen-rich saline were noted [30]. In another study, an increase in pro-inflammatory cytokines was associated with the release of ROS [12]. Thus, our data showing an increase in lipid peroxidation (Figure 2) points to a role for oxidative stress in hapten-evoked, atopic dermatitis pathology.
Thus, we successfully used the dermatitis model and found the H2-releasing device has a therapeutic effect on skin damage and can improve dermatitis-like symptoms in hairless mice. However, further research needs to be extended to longer-term use of oxazolone because we did not achieve the profound immunological changes associated with chronic dermatitis. This was clear from the rapid recovery of the animals after cessation of oxazolone irritation. The rapid recovery suggests we induced an acute rather than chronic condition, even though we followed protocols and schemes proposed for induction of experimental dermatitis in SKH-1 mice and other mouse strains [11-13,15].
The advantage of the transdermal device is its convenience and high degree of H2 delivery to target sites. The device delivers comparable levels of H2 as bathing the skin in hydrogen-rich water. Only, bathing in hydrogen-rich water requires massive volumes and laborious prolonged skin exposure [3]. Treatment of skin conditions can also be achieved by injections of hydrogen-saturated saline, but this method suffers from systemic side effects because the administration method is invasive [30]. In that study, H2 concentrations in tissues were evaluated using the hydrogen microelectrode. Remarkably, our method of H2 administration using the developed device on skin achieved levels comparable to injection. Thus, we have achieved efficient, non-invasive delivery of H2 to tissue with minimal side effect.
In some ways, human studies will be easier and may have greater success than using the mouse model because the mice tend to remove the device, which had to be tightly secured. This hurdle will be minimal in the treatment of humans.
Declarations
Author contributions: VK conceived, designed and conducted experiments and analyzed data. DC conceived study and participated in preparation of the Animal Use Protocol and manuscript. SR conceived, designed and conducted experiments; analyzed data; and wrote manuscript. All authors approved final version.
Conflict of interest: The authors confirm that this article content has no conflict of interest.
Acknowledgements: This work was supported by the SMU overhead recovery fund to Svetlana Radyuk and Daniel Crow’s personal funds. The experiments were conducted at the Laboratory Animal 6 Resource Center (LARC), Southern Methodist University. The authors thank Courtney Yates, SMU LARC manager, for invaluable technical assistance at animal facility.
References
- Kalantar-Zadeh K, Berean KJ, Burgell RE, Muir JG, Gibson PR. Intestinal gases: Influence on gut disorders and the role of dietary manipulations. Nat Rev Gastroenterol Hepatol. 2019; 16: 733-747.
- Shin MH, Park R, Nojima H, Kim HC, Kim YK, et al. Atomic hydrogen surrounded by water molecules, H(H2O)m, modulates basal and UV-induced gene expressions in human skin in vivo. PLoS One. 2013; 8: e61696.
- Zhu Q, Wu Y, Li Y, Chen Z, Wang L, et al. Positive effects of hydrogen-water bathing in patients of psoriasis and parapsoriasis en plaques. Sci Rep. 2018; 8: 8051.
- Shen M, Zhang H, Yu C, Wang F, Sun X. A review of experimental studies of hydrogen as a new therapeutic agent in emergency and critical care medicine. Med Gas Res. 2014; 4: 17.
- Ohno K, Ito M, Ichihara M, Ito M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxid Med Cell Longev. 2012; 2012: 353152.
- Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010; 44: 971-982.
- Ge L, Yang M, Yang NN, Yin XX, Song WG. Molecular hydrogen: a preventive and therapeutic medical gas for various diseases. Oncotarget. 2017; 8: 102653-102673.
- Ohta S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim Biophys Acta. 2012; 1820: 586-594.
- Chapman JR, Ruben Z, Butchko GM. Histology of and quantitative assays for oxazolone-induced allergic contact dermatitis in mice. Am J Dermatopathol. 1986; 8: 130-138.
- Man MQ, Hatano Y, Lee SH, Man M, Chang S, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. The Journal of investigative dermatology. 2008; 128: 79-86.
- Kim MS, Pyun HB, Hwang JK. Panduratin A, an activator of PPAR-alpha/delta, suppresses the development of oxazolone-induced atopic dermatitis-like symptoms in hairless mice. Life Sci. 2014; 100: 45-54.
- Chang TM, Yang TY, Niu YL, Huang HC. The Extract of D. dasycarpus Ameliorates Oxazolone-Induced Skin Damage in Mice by Anti-Inflammatory and Antioxidant Mechanisms. Antioxidants (Basel). 2018; 7.
- Wu XX, Siu WS, Wat CL, Chan CL, Koon CM, et al. Effects of topical application of a tri-herb formula on inflammatory dry-skin condition in mice with oxazolone-induced atopic dermatitis. Phytomedicine. 2021; 91: 153691.
- Kim MS, Kim JE, Yoon YS, Seo JG, Chung MJ, Yum DY. A Probiotic Preparation Alleviates Atopic Dermatitis-Like Skin Lesions in Murine Models. Toxicol Res. 2016; 32: 149-158.
- Lin CY, Hsieh YT, Chan LY, Yang TY, Maeda T, et al. Dictamnine delivered by PLGA nanocarriers ameliorated inflammation in an oxazolone-induced dermatitis mouse model. J Control Release. 2021; 329: 731-742.
- Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014; 2014: 360438.
- Osikov MV, Davydova EV, Kaigorodtseva NV, Fedosov AA, Il’inykh MA, et al. Influence of Rectal Ozone Application on the Intensity of Free Radical Destruction of Lipids and Intestinal Proteins in the Dynamics of Experimental Colitis. Bull Exp Biol Med. 2022; 173: 24-27.
- Yang J, Zhou WW, Shi DD, Pan FF, Sun WW, et al. The Interaction between Oxidative Stress Biomarkers and Gut Microbiota in the Antioxidant Effects of Extracts from Sonchus brachyotus DC. in Oxazolone-Induced Intestinal Oxidative Stress in Adult Zebrafish. Antioxidants (Basel). 2023; 12.
- Wei Y, Du X, Guo Y, Chang M, Deng B, et al. Elucidation of physicochemical properties of polysaccharides extracted from Cordyceps militaris fruiting bodies with different drying treatments and their effects on ulcerative colitis in zebrafish. Front Nutr. 2022; 9: 980357.
- Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. J Dermatol Sci. 2009; 53: 10-18.
- Kim B, Kim HS. Novel peptide inhibits inflammation by suppressing of protease activated receptor-2. Eur J Pharmacol. 2018; 832: 25-32.
- Yoon KS, Huang XZ, Yoon YS, Kim SK, Song SB, et al. Histological study on the effect of electrolyzed reduced water-bathing on UVB radiation-induced skin injury in hairless mice. Biological & pharmaceutical bulletin. 2011; 34:1671-1677.
- Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014; 144: 1-11.
- Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015; 555: 289-317.
- Yoon YS, Sajo ME, Ignacio RM, Kim SK, Kim CS, et al. Positive Effects of hydrogen water on 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice. Biological & pharmaceutical bulletin. 2014; 37: 1480-1485.
- Kajisa T, Yamaguchi T, Hu A, Suetake N, Kobayashi H. Hydrogen water ameliorates the severity of atopic dermatitis-like lesions and decreases interleukin-1beta, interleukin-33, and mast cell infiltration in NC/Nga mice. Saudi Med J. 2017; 38: 928-933.
- Mei K, Zhao S, Qian L, Li B, Ni J, Cai J. Hydrogen protects rats from dermatitis caused by local radiation. J Dermatolog Treat. 2014; 25: 182-188.
- Guo Z, Zhou B, Li W, Sun X, Luo D. Hydrogen-rich saline protects against ultraviolet B radiation injury in rats. J Biomed Res. 2012; 26: 365-371.
- Corsini E, Galbiati V, Nikitovic D, Tsatsakis AM. Role of oxidative stress in chemical allergens induced skin cells activation. Food Chem Toxicol. 2013; 61: 74-81.
- Zhao L, Wang YB, Qin SR, Ma XM, Sun XJ, et al. Protective effect of hydrogen-rich saline on ischemia/reperfusion injury in rat skin flap. J Zhejiang Univ Sci B. 2013; 14: 382-391.