Review Article
Volume 2, Issue 6
UHRF1: A Diagnostic and Prognostic Marker of Cancer
Qurat-ul-Ain1; Mehak Nimra2; Areej Ghouri3; Shafaq Zahoor4; Asma Sarfraz5; Faiza Naseer5*
1Department of Hamdard University, Islamabad, Pakistan.
2Department of National Institute of Health, Islamabad, Pakistan.
3Department of Quaid-e-Azam University, Islamabad, Pakistan.
4Department of Riphah International University, Islamabad, Pakistan.
5Department of Shifa Tameer e Millat University, Islamabad, Pakistan.
Corresponding Author :
Faiza Naseer
Email: faiza.naseer@ymail.com
Received : May 22, 2023 Accepted : Jun 12, 2023 Published : Jun 19, 2023 Archived : www.meddiscoveries.org
Citation: Ain QU, Nimra M, Ghouri A, Zahoor S, Naseer F, et al. UHRF1: A Diagnostic and Prognostic Marker of Cancer. Med Discoveries. 2023; 2(6): 1046.
Copyright: © 2023 Naseer F. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
There are many members of the UHRF family including UHRF1, UHRF2, UHRF3, and UHRF4 having different functions. In this review, we mainly study the activity of UHRF1. It is a multi-domain protein associated with epigenetic mechanisms of cell regulation and proliferation. UHRF1 stands for Ubiquitin-like PHD Ring finger 1 having a location in the chromosomal region 19p13.3 [1]. It plays a key role in transferring methylation from mother to daughter DNA strands [2-6]. mUHRF1 was discovered against murine thymic lymphoma by engineering antibodies. hUHRF1 has activity of E3 ligases for histones H3.
Expression of UHRF1 increases in breast cancer, cervical lesions, rhabdomyosarcoma, pancreatic adenocarcinoma, prostate cancer, and lung cancer [7]. Increased expression of UHRF1 in human pulmonary fibroblasts causes increased topoisomerase IIα expression and hence increased cell proliferation. Similarly, depletion of UHRF1 causes DNA damage response, G2/M phase arrest, and apoptosis formation [8-10]. UHRF1 by interacting with DNMT1 and HDAC1 induces heterochromatin structure.
Structure of UHRF1
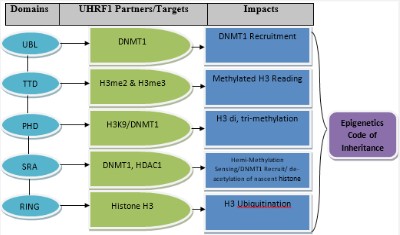
It possesses a UBL (Ubiquitin-like domain), TTD (cryptic Tandem Tudor domain), PHD (plant homeodomain), SRA (Set and Ring associated), and RING (Really Interesting New Gene) domain as shown in figure 1.
UBL domain contains α/β ubiquitin folds with surface Lysine residues i.e. 35% similar to ubiquitin, consisting of 76 amino acids and has an important role in cell cycle progression, protein degradation, and gene transcription [11]. PH Domain recognizes di and tri-methylation of histone h3 lysine 9 (H3K9), associated with heterochromatin formation, transcriptional processes and also with downregulation of UHRF1 both in human and mouse causes disrupt H3K9 distribution. PHD promotes gene activation and inactivation by interacting with tri-methylated H3K4. Various studies have shown that PHD has the ability to read the histone code as well [12]. The SRA domain contains 150-170 amino acids directly involved in DNA methylation to the target sequences by recognizing hemimethylated Cytosine of new daughter DNA strand binds with DNMT1 and involved in heterochromatic formation along with PH domain. It sets a bridge between DNA methylation and histone code by allowing UHRF1 to bind with HDAC1, methylated DNA and DNMT1. RING is 76 amino acid long polypeptide domain, attached with Lysine on cellular protein by its C-terminal. Ubiquitination is mediated by E1/ E2/E3 enzymes; especially E3 catalyzes the binding of the C-terminal with Lysine on targeted protein. There are two main classes of E3 ligases, HECT and Ring class having Ring finger domain [13]. UHRF family has auto-ubiquitinating activity [9] like many ligases which contain Ring finger domain [14]. UHRF1 ubiquitinates histone H3 and these are substrates for the UHRF family.
Mechanism of UHRF1 in heterochromatin formation
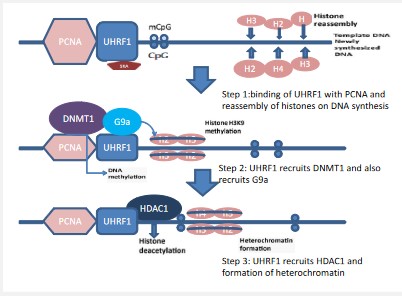
1- First UHRF1 binds to PCNA and recognizes hemimethylated DNA through its SRA domain DNA replication occurs and histones are reassembled immediately on the DNA strand shown in step 1 figure 2 [8,4,6].
2- Secondly UHRF1 recruits the G9a which methylates the h3k9 (histone h3 lysine9) and methylated h3k9 binds with the PHD domain of the UHRF1 Step 2 in figure 7 [15] then it recruits the DNMT1which methylates both DNA strands and transfers the methylation status from mother to daughter DNA strand.
3- Finally UHRF1 recruits the histone deacetylase 1 (HDAC1) which deacetylates the histone proteins and helps in heterochromatin formation and transcriptional suppression Step 3 in figure 2 [15].
UHRF1 and epigenetic codes
UHRF members directly influence the histone code by their enzymatic activity (E3 ligase) via RING, maintaining the epigenetic code (DNA methylation and histone code) and genomic integrity. It might be a tumor suppressor gene [16].
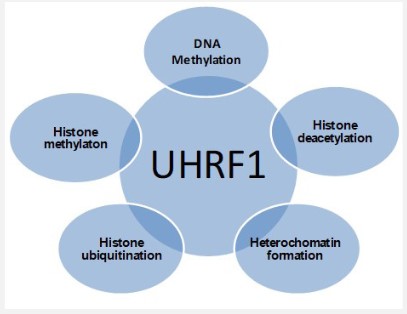
UHRF1 interacts with the methyl-CpG region in DNA strand, methylated H3K9, DNMT1, HDAC1, PCNA and G9a, links with DNA methylation and histone methylation, deacetylation and ubiquitination with heterochromatin formation as shown in figure 3 [4,6,2,1,17,15,18]. DNA methylation and histone modification both act together, changing the gene expression and heterochromatin structure [4,19,20].
Up regulation of UHRF1
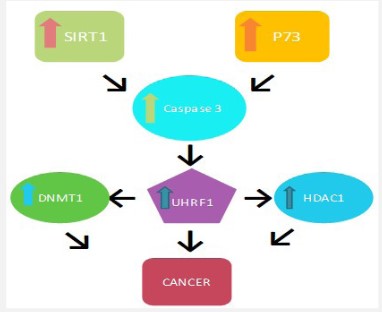
UHRF1 is significantly over-expressed in various cancers and tumor cells [9,21]. Upregulation of UHRF1 is associated with high levels of p73, SIRT1, Caspase 3, DNMT1, and HDAC1 level and ultimately leads to cancer as shown in figure 4. UHRF1 when associated with DNMT1 maintains the methylation status of daughter DNA and maintains the epigenetic inheritance [2,22]. UHRF1 interacts with HDAC1 and transfers them to the methylated tumor suppressor genes [23]. It also maintains histone deacetylation and histone methylation [24] and plays many roles in cancer proving it beneficial for therapeutic targeting [1,25].
Downregulation of UHRF1
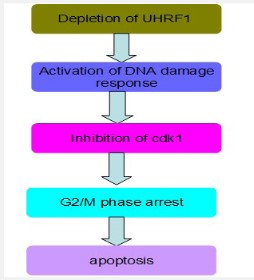
Downregulation of UHRF1 causes DNA damage by breaking DNA strands [26,27], cell cycle arrest in the G2/M stage by inhibiting Cyclin-dependent kinases 1 (cdk-1), a regulator of cell cycle progression in G2 phase during mitosis and apoptosis by activating Caspase 8 [10] as shown in figure 5. chk 1 and chk 2 are activated by phosphorylation in DNA damage response. Loss of chk 2 occurs in UHRF1-depleted cells and inhibits the phosphorylation of chk 1, it leads to cell death by cell cycle arrest. Apoptosis results from depletion of UHRF1 are p53 independent and this pathway regulates Caspase 8 functions, which causes activation of Caspase 3 and ultimately apoptosis [28]. Various studies have shown that depletion of UHRF1 prevents cell cycle progression so it is useful to prevent the growth of tumor cells in cancer treatment [29], and UHRF1 depleted cells become more sensitive to DNA damaging agents [16,30].
Role of UHRF1 in cancers
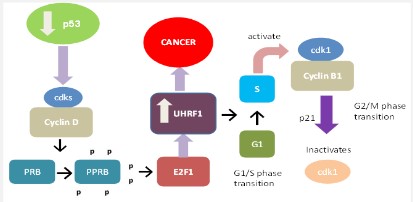
UHRF1 is downregulated by p53 via the up-regulation of p21 and deactivation of E2F1, an up-regulator of UHRF1 [8,21,30]. As p53 is deficient in 50% of cancers [31], So UHRF1 is upregulated in many cancers by the following mechanism. In p53 deficient cancers, the cyclin D/cdks (Cyclin-dependent kinases) complex become activated, causing phosphorylation of PRB and phosphorylated PRB activates the E2F1 which binds with UHRF1 and upregulates its level which leads to cancer as shown in figure 6. UHRF1 is upregulated by rapid cell cycle progression as well [21,8,9,32,33]. In the rapid cell cycle, UHRF1 binds with newly synthesized DNA with PCNA, DNMT1, and HDAC1 and hastily transfers the CH3 group from mother to daughter progeny. It activates the G1/S phase and the cyclin B/cdk1 complex becomes activated in the G2/M phase and p21 inactivates this cdk1 [34].
UHRF1 as a diagnostic and prognostic marker
Up-regulation of UHRF1 has been associated with various cancers e.g, including breast cancer, lungs cancer, bladder, prostate, cervical, and pancreatic cancers [21,33,9,35,32,36]. Up-regulation of UHRF1 is associated with the downregulation of p53 as it has already been discussed in the previous paragraph and it is also essential for cell cycle progression so UHRF1 downregulation is associated with cell growth suppression. It has been used as a diagnostic or prognostic marker by quantitative analysis of UHRF1 in urine and tissue samples of different cancer patients. UHRF1 and other proteins’ expression can be detected by immune histochemistry [8].
UHRF1 as a potential therapeutic target
All UHRF1 members have ubiquitin ligase E3 activity, targeting E3 ligases proved beneficial as it is an ideal drug target in anticancer therapy [13]. Expression of E3 ligases along with UHRF1 increases in cancer cells so when they are inhibited, growth arrest and apoptosis occur [13,11,37]. So, targeting UHRF1 is selective anticancer therapy.
DNA methylation pattern in UHRF1
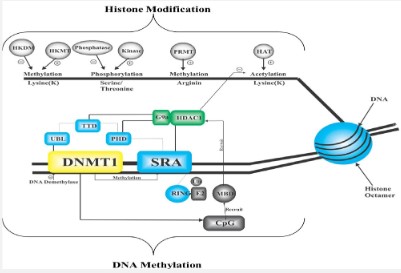
The most important domain involved in DNA methylation is the UHRF1-SRA domain. UHRF1 binds with hemimethylated DNA with the SRA domain which is involved in the proper setting of DNMT1 on the DNA strand [22,4]. DNMT1 and UHRF1 are two proteins that have affinity and selectivity for hemimethylated DNA on their own and both are essential for performing their functions [6]. It is supposed that UHRF1 moves along DNA strands, recognizes hemimethylated CpG region [6,3,4] and it dictates DNMT1 through an unknown mechanism to catalyze methylation at the target site. Another model states that when DNMT1 starts acting, UHRF 1 gets separated from the CpG region, allowing DNMT1 to work properly. It is widely researched how DNA hypermethylation makes the gene transcriptionally silent. DNA methylation behaves as a signal for the recruitment of CpG methyl binding domain (MBD) [38] which further recruits the histone deacetylase (HDAC) and form many genes silencing complexes as shown in Figure 7.
Factor influencing DNA methylation
Many factors have a direct impact on the extent of DNA methylation pattern.
1. Aging: As the tissue becomes aged, there is more possibility that the genome will become hypomethylated and certain CpG islands become hypermethylated. But it is not known whether this change makes the person more susceptible to cancer or not [39].
2. Diet: DNA methylation requires methyl group which comes from folate and methionine components of the diet. As mammals lack the ability to produce folate and methionine themselves, they are totally dependent on diet for carrying out DNA methylation. A diet containing a low amount of these substances causes decrease DNA methylation and increases the tendency of cancer [40].
3. Environment: DNA methylation is also affected by agents such as Arsenic and Cadmium. Ras gene hypomethylation is caused by Arsenic and total DNA hypomethylation is caused by Cadmium which does so by inactivating DNMT1 [40-44].
UHRF1 is a drug able to target cancer therapy
There are 3 ways by which UHRF1 acts as drug able target in cancer therapy;
1- Inhibition of UHRF1 expression inhibits the cells from entering into the S phase and thus it causes growth arrest [30,8,9]. Over-expression of UHRF1 overcomes cell contact inhibition in human lung fibroblasts. Downregulation of UHRF1 activates the DNA damage response and causes cell arrest and apoptosis [16].
2- UHRF1 members are ubiquitin E3 ligases and inhibiting the proteosomes pathway is one of the strategies in anticancer drug development [11].
3- E3 ligases are over-expressed in many cancers and inhibition of E3 ligases causes the growth inhibition of cells and apoptosis in cancer cells [11,13,37]. So, E3 ligases can also be targeted to avoid unwanted effects.
It would be interesting to find a direct inhibitor of UHRF1, it would be helpful because it directly inhibits the cancer cells’ growth and causes apoptosis. Therefore, research on the direct inhibition of SRA and RING domain with its E3 ligase activity is under consideration.
UHRF1 is an attractive potential therapeutic target
It has been shown that with chemical stimulation of double-stranded breaks in DNA by an anticancer agent (Adriamycin) in HCT116 cells (colon cancer), there is a decrease in UHRF1 expression at the transcriptional level and protein [30]. This fall, required for the cells moving towards apoptosis, is controlled by the way p53/p21WAF1/CIP1. By cons, when p53 is defective, there is no decline in the expression of UHRF1. Consequently, one might consider that the lack of decrease in the expression and/or over-expression of UHRF1 in cancer cells results from an alteration of the p53 tumor suppressor gene.
When interferes with the ubiquitin ligase activity of UHRF1 (by overexpression of RING domain mutant sound) increases the sensitivity of cancer cells to chemotherapeutic agents [9]. In addition, it was reported that inhibition of the expression of UHRF1 causes a reduction of ribonucleotide reductase, an enzyme essential for the synthesis of deoxy nucleotides [28]. The concomitant decrease in the expression of both proteins leads to increased cell sensitivity to hydroxyurea (HydréaTM), which can be particularly effective in the treatment of leukemia. It was suggested that the anti-transcriptional targeting of UHRF1 might be interesting in the case of cancers resistant to hydroxyurea without resorting to the increase in therapeutic doses [28].
All these studies seem to converge on the fact that UHRF1 is an attractive target to develop new anti-cancer molecules.
References
- Unoki M. Current and potential anticancer drugs targeting members of the UHRF1 complex. 2008.
- Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007; 450: 908-912.
- Arita K, AriyoshiM, Tochio H, Nakamura Y, Shirakawa M. Recognition of hemimethylated DNA by the SRA protein UHRF1 by a base-flipping mechanism. Nature. 2008; 455: 818-821.
- Hashimoto H, Horton JR, Zhang X, Bostick M, Jacobsen SE, et al. The SRA domain of UHRF1 flips 5-methylcytosine out of the DNA helix. Nature, 2008; 455: 826-829.
- Christian B, Guy F, Frédéric LC, Marcella M, Sirano DP. UHRF1 Links the Histone Code and DNA Methylation to Ensure Faithful Epigenetic Memory. Inheritance, Genetics & Epigenetics. 2009; 2: 29-36.
- Avvakumov GV, Walker JR, Xue S, Li Y, Duan S, et al. Structural basis for recognition of hemimethylated DNA by the SRA domain of human UHRF1. Nature. 2008; 455: 822-825.
- Bronner C, Achour A, Arima Y, Chataigneau T, Saya H, et al. The UHRF family: oncogenes that are drugable targets for cancer therapy in the near future? Pharmacol Ther. 2007; 115: 419-434.
- Unoki M, Nishidate T, Nakamura Y. ICBP90, an E2F-1 target, recruits HDAC1 and binds to methyl-CpG through its SRA domain. Oncogene. 2004; 23: 7601-7610.
- Jenkins Y, Markovtsov V, Lang W, Sharma P, Pearsall D, et al. Critical role of the ubiquitin ligase activity of UHRF1, a nuclear RING finger protein, in tumor cell growth. Mol Biol Cell. 2005; 16: 5621-5629.
- Tien AL, Senbanerjee S, Kulkarni A, Mudbhary R, Goudreau B, et al. UHRF1 depletion causes a G2/M arrest, activation of DNA damage response and apoptosis. Biochem J. 2011; 435: 175-85.
- Sun Y. Targeting E3 ubiquitin ligases for cancer therapy. Cancer Biol Ther. 2003; 2: 623-629.
- Shi Y. Mechanisms of caspase inhibition and activation during apoptosis. Mol. Cell. 2002; 9: 459-470.
- Chen C, Seth AK, Aplin AE. Genetic and expression aberrations of E3 ubiquitin ligases in human breast cancer. Mol Cancer. 2006; 4: 695-707.
- Sambucetti LC, Fischer DD, Zabludoff S, Kwon PO, Chamberlin H, et al. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and anti-proliferative effects. J Biol Chem. 1999; 274: 34940-34947.
- Kim JK, Esteve PO, Jacobsen SE, Pradhan S. UHRF1 binds G9a and participates in p21 transcriptional regulation in mammalian cells. Nucleic Acids Res, 2009; 37: 493-505.
- Muto M, Kanari Y, Kubo E, Takabe T, Kurihara T, et al. Targeted disruption of Np95 gene renders murine embryonic stem cells hypersensitive to DNA damaging agents and DNA replication blocks. J. Biol. Chem. 2002; 277, 34549-34555.
- Karagianni P, Amazit L, Qin J, Wong J. ICBP90, a novel methyl K9 H3 binding protein linking protein ubiquitination with heterochromatin formation. Mol Cell Biol. 2008; 28: 705-717.
- Achour M, Jacq X, Ronde P, Alhosin M, Charlot C, et al. The interaction of the SRA domain of ICBP90 with a novel domain of DNMT1 is involved in the regulation of VEGF gene expression. Oncogene. 2008; 27: 2187-2197.
- Delaval K, Govin J, Cerqueira F, Rousseaux S, Khochbin S, et al. Differential histone modifications mark mouse imprinting control regions during spermatogenesis. EMBO J. 2007; 26: 20-29.
- Vaissière T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008; 659: 40-8.
- Mousli M, Hopfner R, Abbady AQ, Monte D, Jeanblanc M, et al. ICBP90 belongs to a new family of proteins with an expression that is deregulated in cancer cells. Br J Cancer. 2003; 89: 120-127.
- Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, et al. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007; 317: 1760-1764.
- Jeanblanc M, Mousli M, Hopfner R, Bathami K, Martinet N, et al. The retinoblastoma gene and its product are targeted by ICBP90: a key mechanism in the G1/S transition during the cell cycle. Oncogene, 2005; 24: 37-45.
- Rottach A, Frauer C, Pichler G, Bonapace IM, Spada F et al. The multi-domain protein Np95 connects DNA methylation and histone modification. Nucleic Acids Res. 2010; 38: 1796-804.
- Xu F, Mao C, Ding Y, Rui C, Wu L, et al. Molecular and enzymatic profiles of mammalian DNA methyltransferases: structures and targets for drugs. Curr Med Chem. 2010; 17: 4052-4071.
- Mistry H, Gibson L, Yun JW, Sarras H, Tamblyn L, et al. Interplay between Np95 and Eme1 in the DNA damage response. Bio- chem Biophys Res Commun. 2008; 375: 321-325.
- Mistry H, Tamblyn L, Butt H, Sisgoreo D, Gracias A, et al. UHRF1 is a genome caretaker that facilitates the DNA damage response to gamma-irradiation. Genome Integr. 2010; 1: 7.
- Afshar G, Jelluma N, Yang X, Basila D, Arvold ND, et al. Radiation-induced caspase 8 mediates p53-independent apoptosis in glioma cells. Cancer Res. 2006; 66: 4223-4423.
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev. Cell Dev. Biol. 1997; 13: 261-291.
- Arima Y, Hirota T, Bronner C, Mousli M, Fujiwara T et al. Downregulation of nuclear protein ICBP90 by p53/p21Cip1/WAF1-dependent DNA damage checkpoint signals contributes to cell cycle arrest at G1/S transition. Genes Cells. 2004; 9: 131-142.
- Hussain SP, Harris CC. Molecular epidemiology and carcinogenesis: endogenous and exogenous carcinogens. Mutat Res. 2000; 462: 311-322.
- Oba-Shinjo SM, Bengtson MH, Winnischofer SM, Colin C, Vedoy CG, et al. Identification of novel differentially expressed genes in human astrocytomas by cDNA representational difference analysis. Brain Res Mol Brain Res. 2005; 140: 25-33.
- Crnogorac-Jurcevic T, Gangeswaran R, Bhakta V, Capurso G, Lattimore S, et al. Proteomic analysis of chronic pancreatitis and pancreatic adenocarcinoma. Gastroenterology. 2005; 129: 1454-1463.
- Brunet J, Pfaff AW, Abidi A, Unoki M, Nakamura Y et al. Toxoplasma gondii exploits UHRF1 and induces host cell cycle arrest at G2 to enable its proliferation. Cell Microbiol. 2008; 10: 908-920.
- Lorenzato M, Caudroy S, Bronner C, Evrard G, Simon M, et al. Cell cycle and/or proliferation markers: what is the best method to discriminate cervical high-grade lesions. Hum Pathol. 2005; 36: 1101-1107.
- Pita JM, Banito A, Cavaco BM, Leite V. Gene expression profiling associated with the progression to poorly differentiated thyroid carcinomas. Br J Cancer. 2009; 101: 1782-1791.
- Sun Y. E3 ubiquitin ligases as cancer targets and biomarkers. Neoplasia. 2006; 8: 645-654.
- Jones PA, Baylin SB.The epigenomics of cancer. Cell. 2007; 128: 683-692.
- Richardson B. Impact of aging on DNA methylation. Ageing Res. Rev. 2003; 2: 245-261.
- Feinberg AP, Tycko B. The history of cancer epigenetics. Nat. Rev. Cancer. 2004; 4: 143-153.
- Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA. 2000; 97: 10014-10019.
- Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007; 8: 983-994.
- Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development, reprogramming and beyond. Nat Rev Genet. 2008; 9: 129-134.
- Szyf M. The DNA methylation machinery as a target for anticancer therapy. Pharmacol Ther. 1996; 70: 1-37.