Research article
Volume 2, Issue 5
Appraisal to Prenatal Ovarian Traits in Morph-Metric Aspects of Nili-Ravi Buffalo Bovine Fetus
Momen Khan1 ; Muhammad Saleem1 ; Farman Ullah2,3*; Zohaib Ahmed Bhutto4 ; Sami Ullah Khan5 ; Muhammad Altaf Hussain4 ; Mohammad Salim6
1Directorate General (Extension) Livestock and Dairy Development Department, Bacha Khan Chowk, Peshawar, Khyber
Pakhtunkhwa, Pakistan.
2Faculty of Veterinary and Animal Sciences, National Center for Livestock Breeding Genetics and Genomics LUAWMS, Uthal,
Balochistan, Pakistan.
3Key Laboratory of Agricultural Animal Genetics, Breeding and Reproduction, Education Ministry of China, College of Animal
Sciences and Technology, Huazhong Agricultural University, Wuhan 430070, Peoples Republic of China.
4Faculty of Veterinary and Animal Sciences, Lasbela University of Agriculture Water and marine sciences (LUAWMS), Uthal
Baluchistan, P/C-90150. Pakistan.
5Faculty Of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia.
6Forestry and Wildlife Management Department, University of Haripur, Haripur, Khyber Pakhtunkhwa, Pakistan.
Corresponding Author:
Farman Ullah
Email: farman_aup@yahoo.com & farman.vas@luawms.edu.pk
Received : Apr 24, 2023 Accepted : May 15, 2023 Published : May 22, 2023 Archived : www.meddiscoveries.org
Citation: Khan M, Saleem M, Ullah F, Bhutto ZA, Khan SU, et al. Appraisal to Prenatal Ovarian Traits in Morph-Metric Aspects of Nili-Ravi Buffalo Bovine Fetus. Med Discoveries. 2023; 2(5): 1042.
Copyright: © 2023 Ullah F. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study aimed to evaluate the Nili-Ravi buffalo changes in fetal growth relationship with crown rump length (C-R) ovarian at fetal ages from 51 to 290 days. In the methods, the weight, size, and other proximate measurements were compared with the ovarian histology of female sex were externally differentiated up to 56 days. In the results, a linear trend in increase between C-R length, age and body weight is observed. The mean weight of prenatal ovary from 51–70-day-old fetus was 18.8 ± 2.33 mg which increased to 163.30 ± 39.00 mg at 271-290 days of fetal age. Linear regression showed highly significant increase with age in right ovarian weight (b=4.89, P <0.001), and left ovarian weight (b=4.79, P <0.001). In conclusion, the novelty of the distinguished age for differentiating the fetus ovary could have critical information of altered ovarian steroidogenesis in a way that might affect sexual maturation in Nili-Ravi Buffalo.
Keywords: Buffalo; Biometrical; Bovine Fetus; Evaluation; Morphological aspects; Nili-Ravi.
Introduction
Among the animals which science has neglected the domestic water buffalo serves as an outstanding example [1]. Scarce information is available regarding the sex differentiation of fetus in buffaloes. [2] reported that the sex of the fetus could be distinguished at 56 days (C-R length, 3-4 cm) of gestation in Iranian buffalo. Also, in Nili-Ravi buffalo, [3] reported sex differentiation at 56 days of gestation. The body weight gain in male fetus of Nili-Ravi buffalo is relatively slow from 96th to 170th days but a sharp increase is observed in weight from 180th to 311th days of life [4]. The development of the fetal ovary has been studied in various mammals, cattle [5], pigs [6], and rats [7]. The weight of the ovary increase steadily with the increase in number of germ cell from 50th day post cotium in cattle [8] and sharp increase is observed in the 9th month of pregnancy [9]. The sudden increase in weight of prenatal ovaries in buffalo from the 8-10th month of gestation coincided with development of attrite vesicular follicles and with increase in maternal plasma FSH level at that time for cattle [10-12]. It has been reported that the development of fetal buffalo ovary is from weight 27 ± 4.3 mg at 3 month to 94.7 ± 39.00 mg at 9 months of gestation [13]. In which, no significant difference between the weights of the right and left ovaries during this period.
[14] reported in Nili-Ravi buffalo a gradual increase in ovarian weight in the early fetal life and rapid increase in later stages of development. [15] mentioned that oocytes were maximum at crown-rump length of 40.5 cm (110th days of gestation); thereafter, oocyte number decreased suddenly in cattle. Primordial follicles were first observed in prenatal development of the Egyptian buffalo ovary at crown-rump length of 6 cm (56 days) [16]. In bovine fetal ovary primary follicles were formed at a crown rump length of 15 cm (86 days) but their number decreased later in pregnancy [17]. Steroid hormone influences the development and maturation of the fetus during intrauterine life and later, the induction of parturition [18]. [19] reported that bovine fetal ovary is steroid on genetically active in utero. At the 9th and 10th month of bovine fetal ovary hormonal activity is indicated by the formation vesicular follicles [20]. The bovine fetal ovary synthesizes and secretes oestradiol-17 beta in vitro, when the sex of embryonic gonads is morphologically distinguishable at 45 ± 3 days: 3.0 to 3.5 cm in crown rump length.
[21] mentioned that cultured bovine fetal ovaries secreted 17-β oestradiol, which measured by radioimmunoassay, from pre-implantation age 32-35 days. 1.8-2.5 crown-rump length (C-R length) and post-implantation embryos (age 36-85 days, 2.6-12.4 cm C-R length). First measurable amounts of 17-β oestradiol ranged from 1.16-2.22 ng/ovary/24h for the 3.3-8.0 cm C-R length, but such hormone is not detectable for 8.1-12.4 cm C-R length. [22] reported that in mixed umbilical cord blood of cow fetus estradiol concentration ranges increase of estradiol plasma concentration observed during the growth of the dominant follicle. A positive correlation between blood flow in the uterine and ovarian arteries and the plasma estrogen concentration during estrus.
The present research work is designed to study the developmental aspect of reproduction in Nili-Ravi buffalo fetus regarding the prenatal ovarian biometry, and histology. This work was initiated in this lab with the aim to develop this aspect of study of development, and to make available preliminary information in this regard.
Materials and methods
Fetuses
Female fetuses of Nili Ravi buffalo were collected for this study from Sihala Abbatoir, Islamabad. After the slaughtering of the animals, these fetuses were collected immediately. These fetuses were from the age of 58 to 290 days. They were grouped in twelve with intervals of twenty days.
Measurements
Fetuses were weighed to the nearest gram and body length was measured from the highest point of the head to the most caudal extent of the tail to the nearest centimeter. (Crown to rump, C-R). The approximate age of fetus was calculated following the method already established in the reproductive endocrinology laboratory, Quaid-i-Azam University.
Y= a + bx
Where Y is C-R length (crown rump length) “a” is the intercept “b” is the regression coefficient “x” is the age. Y = -11.27 + 0.3063 x.
Collection of ovaries
A “V” shaped incision from umbilical cord to the caudal end of the body on ventral side was given to expose and dissect out the ovaries. Ovaries were weighed to nearest milligram and their length and width was measured in millimeters. After this the selected ovaries were fixed for histological studies, while others were stored at -20 Centigrade in deep freezer.
Histology
Ovarian histology of fetuses at the age of 2, 3, 4, 5, and 6 months was carried out. Histology consists of fixation and staining of tissues. After dissection ovaries were fixed in sera for 4-5 hours. Composition of sera was as follows: 60 mL Absolute alcohol, 30 mL Formaldehyde, and 10 mL Glacial acetic acid. Then the tissues were dehydrated as below in ascending grades of propanol as follow: 70% propanol (4-5 h), 80 % propanol (overnight), 90% propanol (2 h), 95 % propanol (2h), and 100 % propanol (4-5 h). After completing dehydration, the tissues were transferred to cedar wood oil until they became clear and transparent. The tissues were then embedded in paraplast by the following process: Benzol I (10 min), Benzol II (10 min), Benzol + paraplast (2 min; 60°C), Paraplast I (morning; 60°C), Paraplast II (overnight; 60 °C), and Paraplast III (morning; 60°C). In the evening the tissues were ready to make blocks. The tissues were sectioned at a thickness of 5-6 um by using Reichert microtome and stretched at 60 degrees centigrade on Fischer slide warmer. After deparaffinization, the slides were transferred to xylol for 15 minutes to remove any remaining wax. The tissues were then hydrated in the descending grades of alcohol, washed in tap water and stained in Harris Hematoxylin. After that the tissues were dehydrated in the ascending grades of alcohol, counter stained with eosin and microphotographed [23].
Statistical analysis
The results were analyzed for significance by analysis of variance test (ANOVA). The variation in length, width and weight of right and left ovary as well as both ovaries of fetal age were studied at an interval of 20 days and thus arranged in twelve groups.
Results
Gross morphology
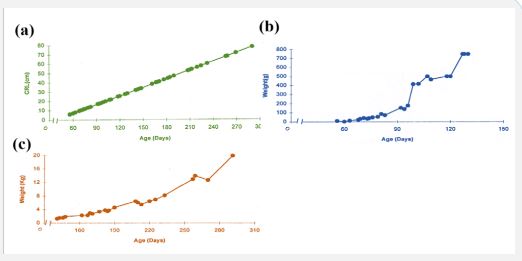
Present study reveals that the sex of the buffalo fetus is externally differentiated at the age of 56 days. Female Nilli Ravi buffalo fetus possesses a pair of ovaries which are situated in the abdominal cavity. Each ovary is an oval shaped structure and appears whitish in color during the early fetal life (130 days) while it becomes pinkish in color as the fetus advances in age. Data on estimated age, body weight, right and left, ovarian weight, as well as both ovarian weights collectively, right, and left ovarian length and width of the Nilli-Ravi buffalo fetuses of different ages are presented in Table 1. Mean ± S.E of fetal length, ovarian weight, length and width increase linearly as the age of the fetus advances are presented in Table 1.
Table 1: Fetal weight with increase in age
| C-R length (cm) | Age (days) | Fetal weight (g) | C-R length (cm) | Age (days) | Fetal weight (g) |
|---|---|---|---|---|---|
| 6 | 56 | 10 | 32 | 141 | 1270 |
| 10 | 69 | 32.6 | 38.5 | 162 | 2250 |
| 10.5 | 71 | 42 | 40 | 167 | 2250 |
| 11 | 73 | 34.67 | 40.5 | 169 | 2930 |
| 11.5 | 74 | 39.74 | 41 | 171 | 2750 |
| 13.5 | 81 | 85.17 | 45 | 184 | 3500 |
| 17.5 | 94 | 138.95 | 52.5 | 208 | 6500 |
| 18 | 96 | 177.68 | 53 | 210 | 6080 |
| 19 | 99 | 413.1 | 54 | 213 | 5500 |
| 27.5 | 127 | 750 | 71.5 | 270 | 12750 |
| 28 | 128 | 750 | 78 | 290 | 20000 |
Body Weight
At the age of 65 ± 1.7 days (C-R length 8.62 ± 0.515 cm) the body weight of the fetus was 21 ± 2.89 g. Body weight increases linearly with age of the fetus, at the age of 290 days body weight of the fetus reaches 20 ± 1 Kg (Table 1, Figure 1, b and c).
Ovarian Weight
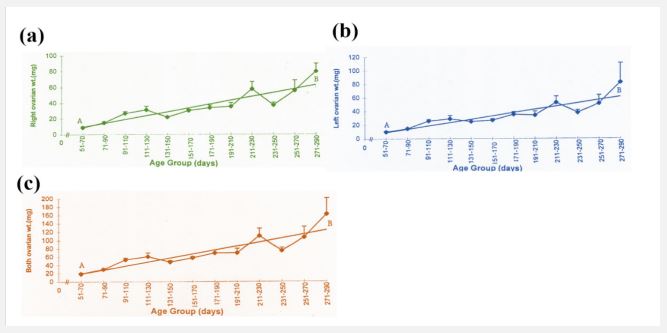
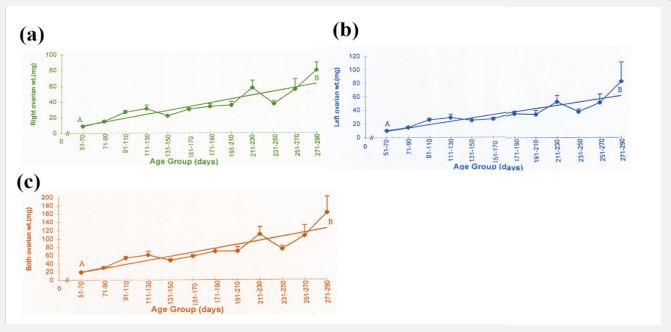
The present study reveals that there is an increase in ovarian weight from 51 days to 290 days of fetal age (Table 2). During this period the mean weight of the right ovary (30.25 ± 2.15 mg), and left ovary (29.59 ± 2.17 mg), and both ovaries (59.82 ± 4.27 mg) is shown in Table I. During the early fetal age (51- 70 days) minimum ovarian weight was observed. An increase in weight is observed up to 110 days in right, left, and both ovaries. From this period onward up to 210 days of fetal age there is no appreciable change but an increase is observed from 211 to 290 days (Table 2, Figure 2 a,b,c) reaching a maximum at 290 days.
Table 2: Nili-Ravi buffalo fetal C-R length, ovarian weight, and size with change in age.
| C-R length (cm) | Age group (days) | Ovarian weight (mg) | Ovarian size (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Both | Right | Left | ||||
| weight (mg) | Length | Width | Length | Width | ||||
| 06-Oct | 51-70 | 8.8 ± 1.46 | 10.00 ± 1.30 | 18.8 ± 2.33 | 3.38 ± 0.23 | 2.05 ± 0.13 | 3.26 ± 0.20 | 2.43 ± 0.17 |
| 10.5-14 | 71-90 | 15.17 ± 1.31 | 14.92 ± 1.05 | 30.08 ± 2.28 | 4.57 ± 0.20 | 3.40 ± 0.24 | 4.57 ± 0.19 | 3.25 ± 0.11 |
| 17-22 | 91-110 | 27.13 ± 2.44 | 26.13 ± 2.15 | 53.22 ± 4.51 | 5.14 ± 0.16 | 3.86 ± 0.14 | 4.86 ± 0.15 | 3.65 ± 0.12 |
| 25-28.5 | 111-130 | 31.67 ± 4.55 | 24.17 ± 4.74 | 60.83 ± 9.23 | 5.04 ± 0.21 | 4.1 ± 0.20 | 5.03 ± 0.20 | 3.75 ± 0.62 |
| 32-34 | 131-150 | 22.25 ± 1.03 | 25.25 ± 3.17 | 47.5 ± 3.79 | 4.68 ± 0.28 | 3.31 ± 0.06 | 4.70 ± 0.17 | 3.15 ± 0.62 |
| 38.5-40.5 | 151-170 | 30.67 ± 1.76 | 27.00 ± 2.08 | 57.67 ± 3.38 | 5.68 ± 0.20 | 4.02 ± 0.15 | 5.55 ± 0.22 | 3.45 ± 0.02 |
| 41-47 | 171-190 | 34.11 ± 3.25 | 35.33 ± 3.91 | 69.44 ± 7.08 | 5.52 ± 0.12 | 3.95 ± 0.10 | 5.61 ± 0.18 | 3.81 ± 0.12 |
| 52.5-53 | 191-210 | 35.75 ± 4.89 | 34.25 ± 5.79 | 70.00 ± 10.64 | 6.13 ± 0.52 | 4.11 ± 0.40 | 5.76 ± 0.49 | 3.65 ± 0.20 |
| 54-57.5 | 211-230 | 58.00 ± 9.07 | 53.00 ± 9.29 | 111.00 ± 17.79 | 6.35 ± 0.30 | 4.15 ± 0.25 | 6.01 ± 0.20 | 4.43 ± 0.02 |
| 60 | 231-250 | 37.5 ± 3.46 | 38.00 ± 4.00 | 75.05 ± 7.5 | 6.47 ± 0.22 | 3.95 ± 0.35 | 6.12 ± 0.37 | 3.95 ± 0.50 |
| 67.5-71.4 | 251-270 | 56.0 ± 12.94 | 51.75 ± 12.47 | 107.75 ± 25.10 | 6.57 ± 0.50 | 4.01 ± 0.28 | 6.75 ± 0.79 | 3.37 ± 0.28 |
| 78 | 271-290 | 80.0 ± 10.00 | 83.00 ± 29.00 | 163.00 ± 39.00 | 8.80 ± 1.20 | 5.15 ± 0.50 | 8.00 ± 0.20 | 4.80 ± 0.70 |
| 30.25 ± 2.15 | 29.59 ± 2.17 | 59.82 ± 4.27 | 5.20 ± 0.13 | 3.71 ± 0.08 | 5.08 ± 0.13 | 3.51 ± 0.08 | ||
The mean weight of left ovary (29.57 ± 2.17 mg) throughout fetal life is not significantly different (P > 0.9) from that of the right ovary (30.25 ± 2.15 mg) and shows the same pattern of increase in weight like right ovary with the advance in fetal age. Similar trend in increase in both ovarian weights is observed. Coefficient of regression was calculated to observe sequential changes of right, left and both ovaries weight with the advance in fetal age. Regression analyses of variance for right, left and both ovarian weight shows a highly significant increase with the advance in fetal age (Table 3).
Table 3: Analysis of variance of regression of right, left and both ovarian weight on fetal age in Nili-Ravi buffalo.
| Organ | Item | d.f. | S.S. | M.S. | F-value | P-value |
|---|---|---|---|---|---|---|
| Right ovarian weight | Linear regression | 1 | 3421.13 | 3421.13 | 38.38 | <0.001 |
| Error | 10 | 891.46 | 89.14 | |||
| Left ovarian weight | Linear regression | 1 | 3291.17 | 3291.17 | 35.69 | <0.001 |
| Error | 10 | 922.03 | 92.2 | |||
| Both ovarian weight | Linear regression | 1 | 1346.71 | 1346.71 | 38.17 | <0.001 |
| Error | 10 | 3527.02 | 352.7 |
P-value of right ovary= 4.89, P-value of left ovary= 4.79, P-value of both ovary= 9.70.
Length and width of ovaries
The mean length and width of the right and left fetal ovary from 51-290 days is 5.20 ± 0.13 mm, 3.17 ± 0.08 mm and 5.08 ± 0.13 mm: 3.51 ± 0.08 mm respectively. The lowest length and width of right and left ovary is observed during 51-70 days of fetal life (Table 2) a slight increase occurs from this stage up to 190 days. Whereas from this stage onward there is gradual increase in length and width of the ovaries up to 270 days of fetal age. During the later fetal period (271-290 days) maximum values are observed (Table 2, Figure 3 a, b; Figure 4 a, b).
Coefficient of regression was calculated to observe sequential changes with the advance in fetal age. Regression analyses of variance for the length and width of right and left ovary showed a highly significant increase with advance in fetal age (Table 4).
Table 4: Analysis of variance of regression of right, left and both ovarian weight on fetal age in Nili-Ravi buffalo.
| Organ | Item | d.f. | S.S. | M.S. | F-value | P-value |
|---|---|---|---|---|---|---|
| Right ovarian length | Linear regression | 1 | 16.68 | 16.68 | 49.76 | <0.001 |
| Error | 10 | 3.356 | 0.334 | |||
| Right ovarian Width | Linear regression | 1 | 2.47 | 2.47 | 13.07 | <0.001 |
| Error | 10 | 1.89 | 0.19 | |||
| Left ovarian Length | Linear regression | 1 | 13.75 | 13.75 | 71.25 | <0.001 |
| Error | 10 | 1.93 | 0.19 | |||
| Left ovarian Width | Linear regression | 1 | 2.08 | 2.08 | 10.46 | <0.001 |
| Error | 10 | 1.99 | 0.19 |
P-value of right ovary length= 0.34, P-value of right ovary width= 0.13, P-value of left ovary length= 0.31, P-value of left ovary width= 0.12.
Histology
Study of the cross section of the ovary during various fetal ages reveals that ovary is lined by thin surface epithelium on its outer periphery; below the surface epithelium tunica albugenia is observed which 4-5 cells in thickness are Tunica albugenia becomes thick with the increase in age of the fetus. Ovary is not distinguishable in cortex and medulla from 61-102 days (C-R length 7.5-20 cm) of fetal life. From 11 days onwards the cortex and medulla are distinct. The cortex possesses variable number of oogonia, oocytes and follicle at different developmental stages. Oogonia have spherical darkly stained nuclei and lightly stained cytoplasm. Oocytes are characterized by mitotic activity of their nuclei. Primordial follicle consists of oocyte and surrounding granulosa cells. The mitotic activity in oogonia is observed at the age 73 days (C-R length 11 cm) which results in the formation of oocytes and these oocytes are present up to the age of 184 days (C-R length 45 cm). The primordial follicles are formed from day 57 onward, but greater numbers of follicles are present between 118 to 210 days.
Age 61 days (C-R Length 7.5 cm)
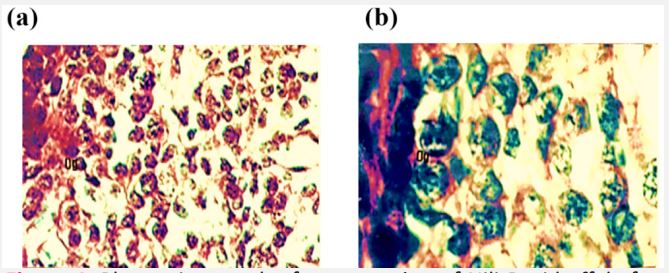
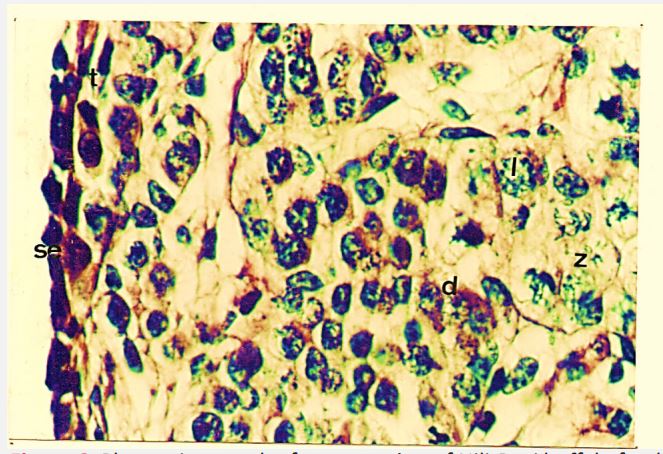
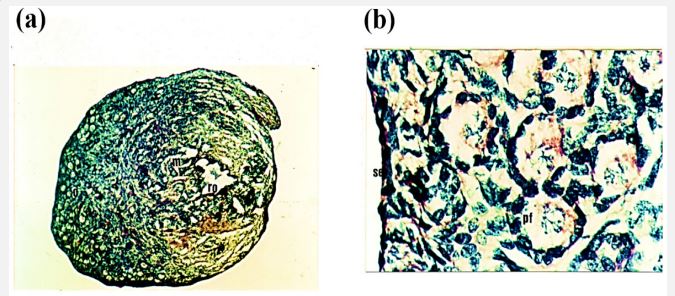
Present study reveals that the ovary at the age of 51 days is not differentiated into cortex and medulla. It is lined by very thin surface epithelium on its outer periphery which consists of flattened cells with darkly stained elongated nuclei. Below the surface epithelium is tunica albugenia which is 4-5 cells thick and possess flattened darkly stained cells (Figure 4).
Ovary is mainly filled with oogonia and somatic cells. The oogonia lies in cell syncytium below the tunica albugenia. While in the middle of the gonad oogonia are scattered through the tissues. The oogonia have spherical darkly stained nuclei and lightly stained cytoplasm (Figure 4a,b). Somatic cells are of variable shapes and sizes.
Age 102 days (C-R Length 20 cm)
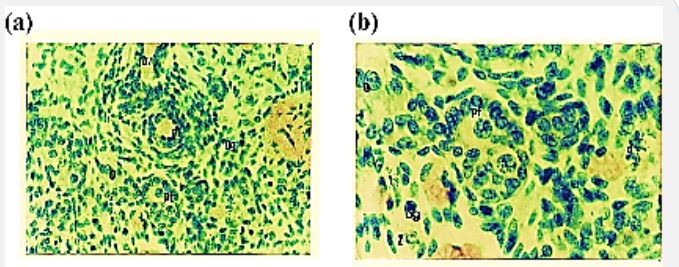
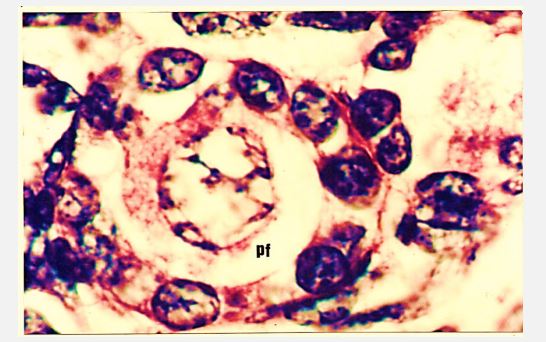
At this fetal age the outer periphery of the ovary is lined by surface epithelium. Cells of the surface epithelium are flattened, darkly stained, and thickened. Size of the tunica albugenia is also increased. Demarcation between cortex and medulla is seen. And it is noted that the medullary region is smaller than the cortex. The cortex is composed of oogonia, oocytes, primordial follicles, and somatic cells. Oogonia are fewer in number as compared to previous stage. Meiotic activity of oogonia like zygotene, leptothene and diplotene are variable. Therefore, oocytes are abundant. Primordial follicles are seen with few granulaosa cells which are elongated while others possess cuboidal shaped granulosa cells. Granulosa cells have darkly stained nucleus. The nucleus of the oocyte within the primordial follicle is spherical and darkly stained while cytoplasm is slightly eosinophilic. Medulla consists of somatic cords, reti ovarii and blood vessels (Figure 5 a,b and Figure 6).
Figure 5: Photomicrograph of cross section of Nili-Ravi buffalo fetus illustrating (a) oogonia (og) and oocyte (o), primordial follicle (pf), and blood vessels (bv) ×112. (b) Higher magnification of (a) portion showing the primordial follicle (pf), Zygotene (Z), diplotene (d) and leptotene (l) H.E ×224.
Age 126 days (C-R Length 27.5 cm)
In this stage cells of the surface epithelium are flattened in shape, having darkly stained nuclei. Tunica albugenia has increased in size. The cortical size has decreased. Oogonia are same in number as in 102 days ovary. However, the number of oocytes has decreased. In comparison to these the primordial follicles are greater in number (Table 5).
Table 5: Changes in the population of oogonia, oocyte and primordial follicle with age.
| C-R length (cm) | Age (days) | Oogonia | Oocyte | Primordial follicle |
|---|---|---|---|---|
| 7.5 | 61 | +++ | - | - |
| 8 | 63 | +++ | - | - |
| 10.5 | 71 | +++ | - | - |
| 11 | 73 | +++ | + | - |
| 13.5 | 81 | +++ | +++ | - |
| 15.5 | 87 | ++ | +++ | + |
| 16 | 89 | ++ | +++ | + |
| 19.2 | 99 | + | +++ | ++ |
| 20 | 102 | + | +++ | ++ |
| 25 | 118 | + | ++ | +++ |
| 27 | 122 | + | ++ | +++ |
| 27.5 | 126 | + | + | +++ |
| 33.5 | 146 | + | + | +++ |
| 34 | 148 | - | + | +++ |
| 36 | 154 | - | + | +++ |
| 38 | 161 | - | + | +++ |
| 42.5 | 176 | - | + | +++ |
| 44 | 180 | - | + | +++ |
| 45 | 184 | - | + | +++ |
| 53 | 210 | - | - | +++ |
| 63 | 242 | - | - | ++ |
| 70 | 265 | - | - | ++ |
| 77 | 288 | - | - | +++ |
Primordial follicles consist of oocyte surrounded by flattened and cuboidal shaped granulosa cells. Most of the follicles are completely encircled by the granulosa cells apart from few which are surrounded by 3-4 granulosa cells (Figure 7a, b).
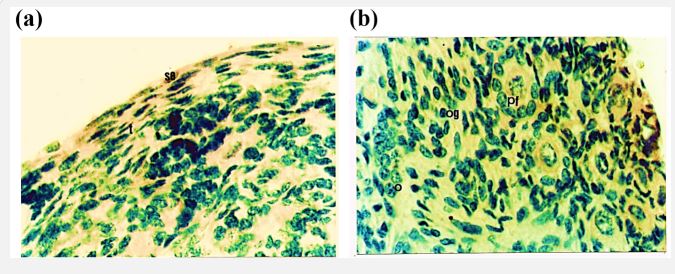
Age 146 days (C-R Length 33.5 cm)
In this fetal age the cellular arrangement of surface epithelium is the same as observed in the previous stage. The size of the tunica albugenia appears slightly increased from the previous age. There is not such change in the organization of oogonia, oocytes and primordial follicles in the cortex. But the number of oocytes has decreased whereas primordial follicles are abundant. At this stage almost all the follicles are completely encircled by granulosa cells (Figure 8a, b).
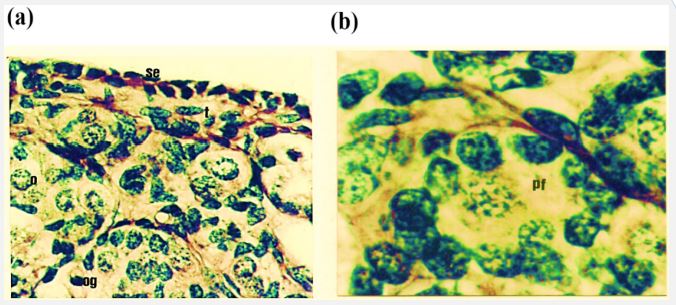
Age 184 days (C-R Length 45 cm)
At this fetal age surface epithelium consists of cuboidal cells with darkly stained nuclei. Tunica albugenia is highly developed and thicker. Cells of the tunica albugenia are loosely arranged elongated and have darkly stained nuclei. The size of the cortex is decreased while medullary area is increased. The Ovarian cortex is full of a greater number of primordial follicles of variable sizes which are completely arranged. Some of the follicles have developed multilayers of granulosa cells while other follicles have single layer of cuboidal shaped granulosa cells (Figure 9a, b and Figure 10).
Discussion
In the present study on Nili-Ravi buffalo an increase in the fetal C-R length and body weight with the increase in age was observed. Similar results were reported by [24] in cattle in Iran. A rapid increase in fetal age, a sharp increase in weight however, is observed from 141 days to 290 days of fetal age. [25] suggested in domestic animal that early slow gain in weight compared to C-R length is because weight is a variable factor. Also, the cattle weight change is not linear but increase with age up to [26,27]. Also, sex differentiation is observed at fetal age of 56 days, prior to this age fetus sex differentiation occurred at the 30th days of gestation [28]. The sex of fetus is distinguished externally at 50 days of gestation in Indian buffalo [29]. In which, the weight of right ovary and left ovary combined together is 59.82 ± 4.27 mg from 50 to 90 days. Also, comparable increases in ovarian weight in Indian buffalo from 2 months (16.45 ± 3.68 mg) to the end of gestation (148.0 ± 20.00 mg) [30,31]. Linear regression shows highly significant increase with age in right ovarian weight (b = 4.89, F = 38.38, P ≤0.001), left ovarian weight (b = 4.79, F = 35.69, P ≤0.001), and both ovaries combined together (b = 9.70, F = 38.17, P ≤ 0.001). [32] reported that the weight of the right ovary (RO) was greater than left ovary (LO) (P < 0.05) with 0.393 ± 0.04 g for RO than LO with 0.355 ± 0.05 g. In Nili-Ravi buffalo ovaries in the 3rd month of fetal age (30.08 ± 2.28 mg) and 9th month of fetal age (163.0 ± 39.00) are heavier compared to Egyptian buffalo in the same fetal ages (3rd months – 27.0 ± 4.3 mg; 9th month 94.7 ± 39.0 mg) [33,16,34].
In Nili-Ravi buffalo fetus length (P ≤ 0.001) and width (P ≤ 0.001) of right and left ovaries increase significantly with the increase in age. This information is, however, not reported for buffalo fetuses from other countries. The histological study in Nili-Ravi buffalo fetal ovary showed that oogonia are abundant at 2 months of age. As the oogonia decreased during the 4th month of fetus and oocytes started appearing in tissue. Also, there are very rare oocytes and abundant oogonia in the ovary of buffalo at the age of 4 month [15]. However, in Nili-Ravi buffalo oogonia from 5th month fetal age onward were not observed. In this study meiosis is observed in the 3rd month of fetal age whereas in cows meiosis starts between 75-80 days of fetal age [14]. In this study differentiation of cortex and medulla is observed at the age of 4th month of the fetus in Nili-Ravi buffalo. Oocytes are mostly towards cortical portion with few primordial follicles towards the cortico medullary region.
The same arrangement of oocytes is observed in the premature mice [34]. Primordial follicles in Nili-Ravi buffalo fetal ovary are from 118-288 days of gestation. [35] also observed similar results in Nili-Ravi buffalo. In the present study prenatal ovary in Nili-Ravi buffalo tunica albugenia become thick with the increase in age of the fetus. [16] reported in camel that tunica albogenia become thick with increase in age. In vitro estrogen production by fetal ovary has been studied in various animals, like rat, and bovine [36,7].
Conclusion
It can be concluded from this study that fetal ovarian and serum estrogen concentration significantly increased with the advancing fetal age, a phenomenon contrary to other mammals. In which, the postpartum can stimulate follicular growth in the buffalo’s fetal ovary. With the introduction of a fertile bull in both groups, the treatment resulted in a better pregnancy rate in the Nili-Ravi Buffalo from the experimental group. However the mechanism of the beneficial effect in multiparous buffaloes remains to be elucidated in further investigations in a larger number of animals.
Highlights
Sex differentiation and changes in fetal growth are related with crown rump length ovarian. Linear regression showed significant increase with age in right ovarian weight (P <0.001). Histology showed that ovaries at 118 days ovary was distinguishable in cortex and medulla. Nili-Ravi buffalo fetal ovary showed that oogonia are abundant at 2 months of age. Oogonia decreased during 4th month of fetus and oocytes started appearing in tissue
Declarations
Acknowledgements: The authors express their sincere thanks to Professor Dr. Samina Jalali for his supervision and guidance.
Data availability: The data used to support the findings of this study are included within the article.
Conflicts of interest: The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Ahmad HI, Ahmad MJ, Jabbir F, Ahmar S, Ahmad N, et al. The Domestication Makeup: Evolution, Survival, and Challenges. Frontiers in Ecology and Evolution. 2020; 8.
- Ranjbar R, Rashidi SH, Alboghobei N, Sadrkhanlo RA, Mazaheri Y. Study of Fetal Sex Determination Based on External Genitalia and Gonadal Diferentiation in the Water Buffaloes of Iran. Asian Journal of Animal and Veterinary Advances. 2007; 2: 223-228
- Abbas W, Bhatti SA, Khan MS, Saeed N, Warriach HM, et al. Effect of weaning age and milk feeding volume on growth performance of Nili-Ravi buffalo calves. Italian Journal of Animal Science. 2017; 16; 490-499.
- Asma UlH, Awan MA, Mehmood A, Sultana T, Shahzad Q, et al. Sperm sexing in Nili-Ravi buffalo through modified swim up: Validation using SYBR((R)) green real-time PCR. Anim Reprod Sci. 2017; 182: 69-76.
- Hartanti MD, Hummitzsch K, Irving-Rodgers HF, Bonner WM, Copping KJ, et al. Morphometric and gene expression analyses of stromal expansion during development of the bovine fetal ovary. Reprod Fertil Dev. 2019; 31: 482-495.
- Yuan Z, Fang Y, Zhang T, Fei Z, Han F, et al. The pomegranate (Punica granatum L.) genome provides insights into fruit quality and ovule developmental biology. Plant Biotechnol J. 2018; 16: 1363-1374.
- Zhang Y, Zhao W, Xu H, Hu M, Guo X, et al. Hyperandrogenism and insulin resistance-induced fetal loss: evidence for placental mitochondrial abnormalities and elevated reactive oxygen species production in pregnant rats that mimic the clinical features of polycystic ovary syndrome. J Physiol. 2019; 597: 3927-3950.
- Galindo J, Galina CS, Estrada S, Romero JJ. Effect of Changes in Body Weight, Body Condition and Back Fat During Last Month of Pregnancy on the Reproductive Efficiency of Bos indicus Cows in the Tropics of Costa Rica. Open Journal of Veterinary Medicine. 2013; 03: 22-28.
- . Mossa F, Ireland JJ. Physiology and endocrinology symposium: Anti-Mullerian hormone: a biomarker for the ovarian reserve, ovarian function, and fertility in dairy cows. J Anim Sci. 2019; 97: 1446-1455.
- Abd-Elnaeim MMM, Miglino MA, Pfarrer C, Leiser R. Microvascular architecture of the fetal cotyledons in water buffaloes (Bubalus bubalis) during different stages of pregnancy. Annals of Anatomy - Anatomischer Anzeiger. 2003; 185: 325-334.
- Dado-Senn B, Laporta J, Dahl GE. Carry over effects of late-gestational heat stress on dairy cattle progeny. Theriogenology. 2020; 154: 17-23.
- Huber E, Notaro US, Recce S, Rodriguez FM, Ortega HH, et al. Fetal programming in dairy cows: Effect of heat stress on progeny fertility and associations with the hypothalamic-pituitaryadrenal axis functions. Anim Reprod Sci. 2020; 216: 106348.
- Balhara AK, Gupta M, Singh S, Mohanty AK, et al. Early pregnancy diagnosis in bovines: current status and future directions. Scientific World Journal. 2013; 958540.
- Sandhu MA, Saeed AA, Khilji MS, Pasha RH, Mukhtar N, et al. Ontogenic development of corticotrophs in fetal buffalo (Bubalus bubalis) pituitary gland. Eur J Histochem. 2014; 58: 2292.
- . Nagina G, Asima A, Nemat U, Shamim A. Effect of melatonin on maturation capacity and fertilization of Nili-Ravi buffalo (Bubalus bubalis) oocytes. Open Vet J. 2016; 6: 128-134.
- . Mostafa TH, Abd El-Salaam AM, Ahmadi EA, Fadel MS, Zeidan AB, et al. Evelopment of some fetal measurements and ovarian hormones during gestation period in maghrebian she-camels under egyptian conditions. Journal of Agriculture and Veterinary Science. 2018; 11: 50-59.
- Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during midgestation and is associated with meiotic arrest of oocytes. Biol Reprod. 2008; 78: 1153-1161.
- Solano ME, Arck PC. Steroids, Pregnancy and Fetal Development. Front Immunol. 2019; 10 : 3017.
- Hartanti MD, Rosario R, Hummitzsch K, Bastian NA, Hatzirodos N, et al. Could perturbed fetal development of the ovary contribute to the development of polycystic ovary syndrome in later life? PLoS One. 2020; 15: e0229351.
- Fortune JE, Yang MY, Allen JJ, Herrick SL. Triennial Reproduction Symposium: the ovarian follicular reserve in cattle: what regulates its formation and size? J Anim Sci. 2013; 91: 3041-3050.
- Sheldon M. Metritis Complex in Cattle. In T. J. P. a. G. C. W. E. David E. Noakes (Ed.), Veterinary Reproduction and Obstetrics Veterinary Reproduction and Obstetrics (Tenth Edition), Elsevier, 2019; 408-433.
- Silva M, Urra F, Ratto M. Uterine endometrial vascularization during ovarian follicular growth in llamas: The effect of estradiol plasma concentration. Theriogenology. 2018; 106: 164-169.
- Sun M, Du XL, Liu JQ, Dahms HU, Wang L. Histological analysis of oogenesis and ovarian development of the freshwater crab Sinopotamon henanense. Tissue Cell. 2018; 53: 37-43.
- Lotfan M, Ali SA, Yadav ML, Choudhary S, Jena MK, et al. Genome-wide gene expression analysis of 45days pregnant fetal cotyledons vis-a-vis non-pregnant caruncles in buffalo (Bubalus bubalis). Gene. 2018; 654: 127-137
- Geiger M, Sanchez-Villagra MR. Similar rates of morphological evolution in domesticated and wild pigs and dogs. Front Zool. 2018; 15: 23.
- Megahed AA, Hiew MWH, El Badawy SA, Constable PD. Plasma calcium concentrations are decreased at least 9 hours before parturition in multiparous Holstein-Friesian cattle in a herd fed an acidogenic diet during late gestation. J Dairy Sci. 2018; 101:1365-1378
- Scholz AM, Bünger L, Kongsro J, Baulain U, Mitchell AD. Noninvasive methods for the determination of body and carcass composition in livestock: dual-energy X-ray absorptiometry, computed tomography, magnetic resonance imaging and ultrasound: invited review. Animal. 2015; 9: 1250-1264.
- Sabetghadam F, Mogheiseh A, Ahmadi N, Khaksar Z, Heidari M. Histo-morphologic study in fetuses and hormonal changes in fetal fluids during sex differentiation of sheep. Small Ruminant Research. 2018; 165: 101-110.
- Franciolli AL, Cordeiro BM, da Fonseca ET, Rodrigues MN, Sarmento CA, et al. Characteristics of the equine embryo and fetus from days 15 to 107 of pregnancy. Theriogenology. 2011; 76: 819-832.
- Sharma BK, Clark AK, Drackley JK, Sahlu T, Schingoethe DJ. Digestibility in Vitro and by Sheep of Sunflower Hulls Treated with Sodium, Potassium and Ammonium Hydroxides. Canadian Journal of Animal Science. 1988; 68: 987-992.
- Sharma NC, Luktuke SN. Incidence of True Anestrus in CrossBred Cows. Indian Journal of Animal Sciences. 1983; 53: 204- 205.
- Dangudubiyyam SV, Ginther OJ. Relationship between more follicles in right than left ovary in recently born calves and right ovary propensity for ovulation in cattle. Reprod Biol. 2019; 19: 363-367.
- Alkafafy M, Sinowatz F. Prenatal development of the bovine epididymis: light microscopical, glycohistochemical and immunohistochemical studies. Acta Histochem. 2012; 11: 4 682-694.
- Yang CY, Zheng HY, Abdelnour SA, Li LY, Shokrollahi B, et al. Molecular signatures of in vitro produced embryos derived from ovum pick up or slaughterhouse oocytes in buffalo. Theriogenology. 2021; 169: 14-20.
- Shahid B, Jalali S, Khan MI, Shami SA. Different Methods of Oocytes Recovery for in Vitro Maturation in Nili Ravi Buffalo’S Oocytes. APCBEE Procedia. 2014; 8: 359-363.
- Cooke PS, Mesa AM, Sirohi VK, Levin ER. Role of nuclear and membrane estrogen signaling pathways in the male and female reproductive tract. Differentiation. 2021; 118: 24-33.