Review article
Volume 2, Issue 4
Biomaterials Surface Engineering Towards Anti-Adhesive Urinary Catheters
Tugce Caykara1,2; Ligia R Rodrigues2; Carla Silva1,3*
1CENTI - Centre for Nanotechnology and Smart Materials, Rua Fernando Mesquita 2785, 4760-034, Vila Nova de Famalicão,
Portugal.
2CEB - Centre of Biological Engineering, Universidade do Minho, Campus de Gualtar, 4710-057 Braga, Portugal.
3CITEVE - Portuguese Technological Centre for the Textile and Clothing Industries, Rua Fernando Mesquita 2785, 4760-034, Vila
Nova de Famalicão, Portugal.
Corresponding Author :
Carla Silva
Email: csilva@centi.pt
Received : Apr 06, 2023 Accepted : May 11, 2023 Published : May 18, 2023 Archived : www.meddiscoveries.org
Citation: Caykara T, Rodrigues LR, Silva C. Biomaterials Surface Engineering Towards Anti-Adhesive Urinary Catheters. Med Discoveries. 2023; 2(5): 1041.
Copyright: © 2023 Silva C. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Hospital Associated Infections (HAI) have been growing in number over the years, representing a concern that requires attention. The most frequent infections are observed in the urinary tract and 80% of them are catheter related. These infections cause problems ranging from mild discomfort to severe implications and even death. Being the bacterial adhesion onto to the material’s surfaces the first step of many of these catheter-related infections, the ability to modify those materials, conferring them anti-adhesive features, is seen as an important strategy towards the prevention and/or reduction of HAI.
Bacterial adhesion is a complex phenomenon mediated by several factors. Bacteria have morphological differences like different shape and cellular wall composition, surface appendages, surface hydrophilicity/hydrophobicity and surface charge and the complexity of bacterial adhesion increases when they interact with material surfaces and under variable environmental conditions. This review focuses on the design of novel materials for urinary catheters based on the factors that affect bacterial adhesion and biofilm formation. Strategies being developed to confer these urinary catheters biodegradability and antimicrobial properties will also be discussed.
Keywords: Biomaterials; Urinary catheters; Anti-adhesive; Surface modification; Bacterial adhesion.
Introduction
Bacterial adhesion is a concern in several fields including hospitals, implants, diagnostic tools, membranes, bioreactors, food and marine industry, among others [1]. In the healthcare sector, dealing with bacterial adhesion and consequent biofilm formation through the design of novel anti-adhesive materials can help to decrease expenditure [2], antibiotic resistance and deaths resulting from material-related infections [2]. Over 90% of implants contain pathogens due to the contact with patients skin, medical personal clothing, and medical equipment, or due to their presence in the patient’s body when implanted [3]. In 2017, it was estimated that 8.9 million registered infection cases occurred in hospitals and long-term care facilities only within Europe [4], while this number in 2012 was estimated to be more than 4 million [2]. As also it can be seen from the infection cases, the global rise of human infectious disease outbreaks is a current well known concern [5].
The most frequent infections are observed in the urinary tract. Urinary tract infections account for 30-40% of total HAI worldwide and 80% of those infections are associated with urinary catheters. Moreover, it is important to notice that these statistics have been drawn for developed countries and that for less developed countries numbers are expected to be 3 – 5 times higher [6]. Both catheters and stents offer a simple method to maintain internal drainage of urinary track. However, these biomedical devices provide an ideal place for bacterial colonisation and biofilms formation leading to infections and playing a critical role in morbidity [7]. Catheters can be used for short-term (up to 7 days) or long-term (28 days or longer) procedures [8]. Indeed, urinary catheters can stay in place for up to 12 weeks, however, this is not the common case due to encrustation and bacterial infection, which can block catheters and cause complications ranging from mild encrustation and bladder stones to severe septicaemia, endotoxic shock and pyelonephritis [9].
Factors that prevent urinary infections in healthy people are (1) the repeated sloughing of urethral epithelial cells, (2) innate mucosal immune function and (3) the periodic flushing action of urine expelled from the bladder. As mentioned, with catheters, the material surface offers a spot for bacterial colonisation, followed by biofilm formation and encrustation [8]. As a solution to overcome this problem, intermitted catheters (IC) can be used, however, not all patients are eligible to use them, particularly those lacking the ability to take care of their own needs. Nevertheless, the occurrence of infections is the main issue arising from catheter’s use independently of being for longterm or short-term procedures. Indeed, all catheter types and brands are somehow susceptible to bacterial adhesion leading ultimately to infections [9].
The infection and bacterial colonisation of the catheters start with the reversible attachment of planktonic bacteria to material’s surfaces. Afterwards, the bacteria can either go back into the bulk medium or strengthen its bond with the surface, resulting in an irreversible adhesion [1]. When proper conditions are provided, the bacteria will divide and form microcolonies, and then, those microcolonies will grow and form a mature biofilm [10]. A biofilm is a microbial community surrounded by a polymer matrix composed of polysaccharides, secreted proteins, lipids and extracellular DNA [11]. However, at this stage, continuous growth of the microbial colonies can become unsustainable due to environmental limitations such as low levels of oxygen and nutrients. Hence, bacteria can be dispersed into the liquid solution and these cells can again attach to (other) surfaces and form new biofilms [10]. Biofilms provide several advantages for bacteria survival including a greater access to nutritional resources, improved survival to biocides, enhanced organism productivity and interactions and greater environmental stability [1]. Bacteria within biofilms are resistant to antibiotics and it has been shown that bacteria collected from both urine and stents are resistant to antibiotics resulting from their biofilm state as can be inferred by the expression of specific biofilm-related genes. Therefore, it is crucial to prevent bacterial adhesion and biofilm formation onto these biomedical devices [12]. Indeed, understanding bacterial adhesion is a key step to further design novel anti-adhesive materials for many applications. In this review, we will discuss the interactions and conditions affecting bacterial adhesion and the design of new materials for urinary catheters.
Forces mediating bacterial adhesion
When bacteria is moving from liquid to a solid surface, Van der Waals, electrostatic (or Coulomb) and acid-base interactions are the first physicochemical forces that bacteria experience [10]. Van der Waals forces are the most long-ranged ones with distances up to 1 µm and these forces become gradually stronger when surfaces become closer [13]. Van der Waals interactions are generally attractive, while electrostatic interactions are modulated by the ionic strength and the pH of the liquid environment, and acid-base hydrophobic interactions can be attractive or repulsive depending on the environment, bacterium and surface chemistries [10]. While Van der Waals interactions are more dominant close to the surface, the electrostatic ones become more dominant at a distance [14]. These bacterial adhesion forces are measured in the picoNewton and nanoNewton ranges [15]. Stronger forces (>10 nN) can cause bacterial cell wall damage and bacterial cell death [13].
Considering the forces applied on a surface, different theories have been developed to explain physicochemical interactions between bacteria and interacting surfaces, namely the Derjaguin-Landau-Verwey Overbeek (DLVO) theory, the thermodynamic theory and the extended DLVO (XDLVO) with thermodynamic approach [16]. However, all these theories do not completely describe adhesion since the bacterial surface is complex, as well as structurally and chemically heterogeneous. Furthermore, extracellular polymeric substances (EPS) and proteinaceous cell appendages bridge between bacteria and the substrate, directly affecting bacterial adhesion and the theories are unable to model it. While EPS is important for biofilm formation, the presence of proteinaceous cell appendages are often essential for the initial bacterial adhesion [17]. Thus, experimental bacterial adhesion studies and design of new antiadhesive materials remain of critical importance [16].
Factors affecting bacterial adhesion
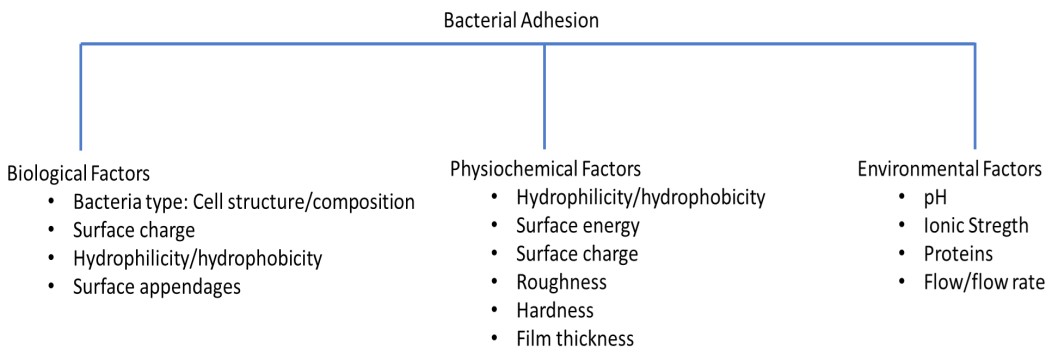
Adhesion of bacteria onto surfaces depends on three main factors (Figure 1), namely bacteria itself (so-called biological factors), material surface (so-called physicochemical factors) and the surrounding environment (so-called environmental factors) [10] which must be considered when engineering materials to confer them anti-adhesive properties.
Biological Factors
Urinary infections are caused by both internal microflora and external contamination [9]. The bacteria most commonly found in catheter-related infections are Enterococcus faecalis, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, Escherichia coli, Citrobacter freundii, Providentia rettgeri, Candida albicans, Morganella morganii, Burkholderia cepacian, Providencia sp., Providencia stuartii [8,9,18]. From all those bacteria, E.coli, E. faecalis, S. epidermidis are frequent in short-term device applications, while P. mirabilis, P. aeruginosa, P. stuartii, M. morgani and K. pneumoniae usually occur in long term applications [18,19]. In indwelling catheters, P. mirabilis biofilm is the main concern, while in intermitted catheters, the main infections that occur are caused by E. coli due to the microflora of meatus being pushed into the bladder [9].
Bacteria can be divided into Gram-negative and Gram-positive depending on the cell wall composition [20]. Although both bacteria types are seen, gram-negative bacteria seem to be the dominant bacteria type in urinary infections [8,9,18]. One of the main differences in these two types of bacteria is the thickness of the peptidoglycan layer in the cell wall. Gram-positive bacteria have a thick peptidoglycan layer above the cell membrane, while gram-negative ones have a thin peptidoglycan layer between the cell membrane and outer membrane layer and it includes a gel-like region which is called periplasmic space [20]. Thus, gram-negative bacteria have a less rigid surface compared to the gram-positive ones and, the more flexible cell wall of gram-negative bacteria can promote a better adhesion onto rough surfaces [21]. Furthermore, the outer cell wall in gramnegative bacteria consists of lipopolysaccharides, whereas in gram-positive bacteria teichoic and lipoteichoic acids in the wall are attached to the peptidoglycan layer [20].
Additionally, bacteria are almost always negatively charged [20]. Bacteria cell surface charge originates from dissociation or protonation of carboxyl, phosphate and amino groups and consequently depends on the environmental pH [22]. The negative bacterial charge is mainly due to phosphate groups in teichoic acids for gram-positive bacteria [23] and phosphoryl and 2-keto3- deoxyoctonate carboxylate groups in gram-negative bacteria [20]. However, the bacterial cell is highly heterogeneous and contains various exposed proteins, lipids and exopolysaccharides, that exhibit different charges and hydrophobicity depending on the growth conditions such as environmental pH and ionic strength, to provide adhesive adaptability [10,24]. Surface charge is also influenced by the bacteria age and surface structure. A high surface charge usually goes together with hydrophilic bacteria [25]. The hydrophobicity of bacteria differs according to the bacterial species and it is also influenced by growth, medium, bacteria age and surface structure [26]. Microbial cell hydrophobicity can be determined by contact angle measurements and the values for some of the abovementioned bacteria can be found in literature [27].
A great variety of structural surface appendages like fimbriae or flagella can be found on the top of the peptidoglycan layer in gram-positive bacteria and on the top of the outer membrane in gram-negative bacteria [22]. The surface appendages are extracellular structures which are important in bacterial growth and survival in diverse environments [28]. Filamentous protein extensions and surface appendages from the cell surface, including flagella, fimbriae, curli and pili, are involved in the nonspecific initial adhesion to abiotic surfaces [10]. On the other hand, non-fibral adhesins on the surface of bacteria can play a role in the close contact between bacterial cell and substrate, as well as in the maturation of interactions and irreversible adhesion [10,24]. It has been shown that adhesins like Fimbriae type 1 (FimA), Fimbriae S (SfaS), PapC (which forms pili) [29] and extracellular polysaccharides [24,30,31] and eDNA (extracellular DNA) [32] also promote adhesion. Moreover, bacterial cells are highly dynamic, and they can adsorb ions and macromolecular components. Charged groups may associate or dissociate upon changes in pH or ionic strength of the surrounding fluid and even when approaching a charged surface. This might induce changes in the conformation of different kinds of surface appendages as fimbriae and flagella [22].
Additionally, bacteria can reach a surface by active and/or passive movement. While some bacteria have swimming motility, some are subjected to physical forces like Brownian motion and gravitational forces to bring them close to the surface. In active movement, bacteria have flagella, which are responsible for the swimming ability by generating a propulsive force. They can also play a role in reversible and irreversible adhesion. Flagella can direct the swimming towards a surface in response to the cues in environment like chemical signals, light, temperature, magnetic fields and oxygen [10,24].
Physicochemical factors
Several physicochemical properties of the surfaces affect bacterial adhesion, namely, wettability, surface energy, surface charge, roughness and others like hardness and film thickness [33,34]. The hydrophobicity effect on bacterial adhesion is mostly governed by the hydrophobicity of the bacterial cell [10]. Hydrophobic bacteria show higher adhesion compared to hydrophilic bacteria, while hydrophilic materials were found to be more resistant to bacterial adhesion than hydrophobic materials. Large numbers of bacteria adhere to hydrophobic surfaces with little or no surface charge, however, this number is smaller for hydrophilic surfaces [26]. The number of bacteria becomes even smaller if the hydrophilic surfaces are negatively charged [25]. A study suggests that bacteria adhere with only few strongly binding macromolecules on hydrophilic surfaces whereas bacteria adhere with many weakly binding macromolecules leading high adhesion with low variability on hydrophobic surfaces. [35]. Anti-adhesive materials via hydrophilic surface modification will benefit from a hydration layer formed on the material surface which acts as a barrier to the bacteria approaching [36]. Although there is an increased interest in hydrophilic surfaces, the bacterial inhibition observed on some natural surfaces like lotus leaves, dragonfly wings and shark skin has inspired the scientific community to also work on the design of superhydrophobic surfaces. These surfaces, due to their superhydrophobic nature, form air pockets on the surface of the materials, preventing bacteria from approaching and consequently adhering [10] and, they are also easier to clean due to a weaker binding at the interface [37].
An additional parameter affecting bacterial adhesion is surface energy [38]. Katsikogianni et al. showed that bacterial adhesion was negatively correlated with the total surface free energy and its polar component according to dispersivepolar approach, where total surface free energy is expressed as the sum of polar and dispersive components [39]. Adhesion free energy becomes more negative resulting in an increased bacterial adhesion strength with the increasing hydrophobicity of a surface [40]. However, some studies have shown that low surface energies (20–30 mN/m) have the lowest adhesion [34]. This may be due to the chemical and physical properties of bacteria, substrates and water solution used [38]. However, since surface energy values are usually calculated using contact angle values, which are an indication of hydrophilicity and hydrophobicity, conflicting results can arise from using these wetting properties of the materials [37].
Another factor influencing bacterial adhesion is the material surface charge. As previously mentioned, bacterial cells generally exhibit a net negative charge. As most surfaces are naturally negatively charged, bacteria experience electric double layer repulsion [34]. However, positively charged surfaces can be used to kill bacteria by attracting them and damaging their cell wall [33].
Roughness is another parameter showing conflicting results [41]. The general opinion in the literature reports that irregularities on polymer surfaces promotes bacterial adhesion, while ultra-smooth surfaces show lower bacterial adhesion. This surface behaviour has been explained with the increase of favourable sites on the surface area for bacterial attachment [34]. However, other studies showed that roughness had no effect or even inhibited the adhesion of bacteria [42] in cases of creating nano roughness [42]. This kind of conflicting results may be due to the bacteria used, as bacteria type (gram type) [21] and fibral structures like flagella [43] can make a difference. Moreover, it should also be noticed that the surface roughness can affect its hydrophilicity and hydrophobicity. Surface wetting can either be homogeneous or heterogeneous, which have different impacts on bacterial adhesion. The Wenzel`s phenomenon suggests that both hydrophilicity and hydrophobicity are enhanced by an increasing roughness on homogenously wetted surfaces, meaning that a hydrophilic surface will become more hydrophilic and a hydrophobic surface will become more hydrophobic [44]. The porous surfaces behave according to another phenomenon, the so-called Cassie-Baxter phenomenon. In this state, the water droplet heterogeneously wets the surface causing air pockets and affecting the wettability [45]. In this way, hydrophobic surfaces can prevent bacterial adhesion.
Additionally, micro-texturing has also been found to be beneficial on surfaces where bacteria width is bigger than the gap between micro-textures. This reduces the bacterial adhesion by reducing the surface area [34]. Thus, it is important to tune the dimensions and shapes of textures according to the bacterial cell to control bacterial cell adhesion [46,47]. Micro and nano texture of the surfaces can also be arranged in a way to mechanically rupture bacterial cell wall, thus providing antibacterial properties while avoiding antimicrobial resistance. However, this mode of action works better for gram-negative bacteria, due to its flexible cell membrane which allows stretching and tearing upon surface adsorption [48].
In addition to the factors discussed above, other factors like hardness and film thickness have attracted attention. While it had been found that increased hardness reduced bacterial cell adhesion in polymers [26,49], there has been reports that soft materials were more resistant to bacterial adhesion [50]. On the other hand, the film thickness was important for soft materials and thicker films reduced bacterial adhesion, which was thought to be due to the stiff substrate having a higher effect on thin films [50].
Environmental factors
Environmental effects caused by the presence of proteins, ions, pH and flow rate can also play a role in bacterial adhesion. Human urine consists of 91–96% water and the remaining parts comprise inorganic salts, urea, organic compounds and organic ammonium salts [51]. It was found that pH and the ionic strength of the surrounding buffer affect the cell surface and material surface’s hydrophobicity [52]. The pH of urine is between 4.5-8 [19]. A catheter problem which arises due to increased pH in the medium is encrustation. It may cause blockage of the catheter leading to damage of the bladder, ureters and kidneys [9]. Encrustation can occur due to metabolic dysfunction but generally, it is due to bacteria as urease-producing bacteria (P. mirabilis, P. vulgaris and P. rettgeri). Urease hydrolyses urea into ammonia and carbon and with increased ammonia, the pH of the urine increases above 8.0, causing calcium and magnesium phosphate to crystallise. However, some urease forming bacteria species do not form crystals due to low levels of urease production. Some of these species include P. aeruginosa, S. aureus, K. pneumoniae, E. coli, M. morganii, and P. stuartii. Nevertheless, other bacteria can still produce mucoid which can also cause catheters blockage. Thus, catheters should be resistant to bacterial adhesion, biofilms formation and encrustation [9]. On the other hand, ionic strength is one of the factors affecting bacterial adhesion. At low ionic strength (≤20 mM) adhesion can be driven by electrostatic repulsion, while Van der Waals and hydrophobic interactions can be primary driving forces at higher ionic strengths (50−100 mM) [14]. The ionic strength of urine is mainly determined by sodium and chloride due to their abundance [53] and its physiological ionic strength is 150 mmol L-1 [54]. Due to the relatively high ionic strength value, it can be said that the adhesion will be driven by Van der Waals and hydrophobic interactions thus, the design of new materials should be more focused on wetting properties of the materials rather than on surface charge. Moreover, other ions like calcium can also enhance bacterial adhesion [14]. While low amount of iron and manganese weaken bacterial adhesion, phosphate limitations enhance bacterial adhesion [10].
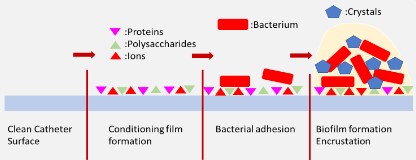
Furthermore, a conditioning film is formed on the surface of the urinary catheter when it enters into the body [19]. This conditioning film consisting of proteins, polysaccharides [55] and urine can make the surface more susceptible to bacterial adhesion [8,56]. Tamm-Horsfall glycoprotein and other proteinaceous molecules like serum albumin, fibrinogen, collagen and fibronectin can be found in conditioning films [57]. Tamm-Horsfall, fibrinogen and fibronectin can promote bacterial adhesion [8,58] while albumin inhibits it [59,60] and collagen has been shown to promote and inhibit bacterial adhesion depending on the experimental conditions [61,62]. Urine also includes proteins from urothelial cells, as well as damages at the urethral lining caused during insertion of catheters [19]. Electrolytes, ions, mineral and other organic molecules will also be present on the conditioning film [19,63–65]. Furthermore, initial bacterial adhesion stages in alkaline environments can cause the formation of microcrystals on the catheter surface which are also proven to support bacterial adhesion (Figure 2) [8,66].
Other important factors to prevent bacteria from reaching and adhering to the surface are flow and shear. If the flow rate is high, it would cause a lower boundary layer, thus bacteria near to the surface are affected by higher shear and at high velocity, causing difficulties in bacteria to approach the surface [10]. Furthermore, antimicrobial substances can be depleted from the surface in a shorter time compared to static conditions. Therefore, catheters should be studied under flow [68]. Unfortunately, the lack of standard testing methods make the development and study of new materials harder to compare, due to absence of well-documented data on bacterial adhesion onto different materials [19,69].
New materials for urinary catheters
Catheters are one of the most commonly used medical devices [9] and bacteria with antibiotic resistant genes found in biofilms and in urinary samples of patients, raises even more concerns [7]. Thus, there is a great need to develop better materials that can prevent or reduce bacterial adhesion, and subsequently biofilm formation and related infections [7].
In order to evaluate these newly designed materials, in vitro testing models have been developed [9,70,71]. However, the evaluation is still a challenge as the urinary systems are complex and inconsistent [75] with several difficulties at mimicking listed in Table 1. Nevertheless, in vitro models are important to test new materials since they can help to avoid ethical issues and higher cost which comes with in vivo tests [9]; however, there is still a need for standard and realistic models to be developed in order to evaluate the performance of the new materials in equivalent conditions.
Table 1: Difficulties at mimicking urinary systems in in vitro studies.
| Factors | Complexities of urinary systems |
|---|---|
| Bacteria | The variation in bacteria types in urine [8,9,18] Strain used with reduced abilities to attach in vitro studies [72] Use of only cultivable strains for in vitro studies [72] Variation in bacteria colonisation time [73] Variation in the number of bacteria [19] |
| Urinary fluid | The difference in nutrients and nutrient levels [72] Lack of Host defence system like antimicrobial proteins and peptides for in vitro models [72] Presence of proteins, cells and crystals in urine [9] Oxygen amount for bacterial growth [75] Other parameters like flow, temperature, osmolarity [68,72] |
| Material | Conditioning film on material [19,55,63–65] Crystal formation on material [8,9,66] Biocompatibility, stability of the material or modification [72] |
Strategies to develop new materials to prevent bacterial adhesion in urinary catheters include mainly (1) incorporating antimicrobial agents and (2) developing antifouling surfaces [33]. The most common commercial products use antimicrobial coatings with silver and antibiotics like nitrofural, sparfloxacin, rifampicin, minocycline, and antifouling coatings by combination with hydrophilic coatings and hydrogels [19,74,75]. However, studies are still being conducted to develop more effective technologies.
Antimicrobial coatings
Antimicrobial studies involve using antimicrobial coatings comprising antibiotics (e.g. rifampicin, triclosan), silver or nanoparticles (carbon nanotube and graphene oxide), enzymes and peptides [8,9,74,76]. Antibiotic coatings are less effective at preventing biofilm formation since urinary tract infections are mostly caused by antibiotic resistant pathogens [9]. Silver is one of the most commonly used antimicrobial agent [74] and it has provided effective results either alone [77] or in combination with other molecules [78]. The combination of silver with antibiotics (amikacin and nitrofurantoin) also showed a synergistic effect by inhibiting bacterial adhesion more effectively compared to the single use of any of them [78]. Although silver plays an important role in the development of antimicrobial catheters, this type of catheter loses its antimicrobial ability in long term uses. The intermittent use can also cause bacterial resistance [74]. Moreover, silver can cause hypersensitive reactions in patients and there is a growing concern about a possible emergence of resistant bacteria [9]. Another bactericide used is nitric oxide which is an endogenously produced bactericidal gas used in urinary catheter studies [79]. Implementation of nitric oxide has shown some better results compared to silver, however there can be side effects like decrease in blood pressure, inhibition in platelet aggregation, increased bleeding, skin irritation, skin edema/erythema and uncontrolled erection [80]. Thus, other alternatives like antimicrobial enzymes [81] and peptides [82] are being investigated as they are less likely to cause the development of bacterial resistance and they may be less toxic to tissues compared to silver [74]. However, their production is more expensive [74,83] and their use might be limited due to enzymatic degradation [84]. Less toxic surfaces with antibacterial properties can also be achieved by the use of natural substances like phenolic compounds, such as vanillic acid which was found to be effective [85], and chitosan which can offer bactericidal and antifouling properties while being cheap and biocompatible [86].
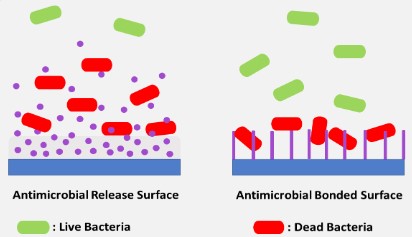
When designing materials, antimicrobials can be incorporated into the surface by using an antimicrobial release approach which affects the material surface and the surrounding or can be bonded to the surface, affecting only the surface of the materials as shown in Figure 3. This incorporation strategy is decided according to the type of antimicrobials incorporated [87]. While some leaching antimicrobials may sensitise patients and cause life-threatening anaphylaxis [87], in other cases surface-bond antimicrobials may lose their efficiency [88]. Surfaces modified with antimicrobials can lose their effectiveness after being implanted since film conditioning and dead bacterial cells can cover the top of the materials preventing the material surface from functioning [72]. Therefore, it is recommended that antimicrobial strategy is implemented along with another strategy.
Antifouling surfaces
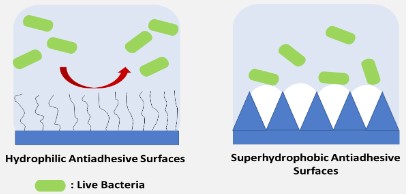
Some other strategies focus on forming anti-adhesive surfaces by modifying the surface physiochemical properties, for example developing hydrophilic and superhydrophobic [74,89] surfaces as shown in Figure 4. Catheters possessing hydrophilic surfaces have been commercially available [8]. Formation of polyethylene glycol brushes (PEG) has been one of the most studied hydrophilic surface modifications [90], being considered the gold standard; however, these modified surfaces are unstable due to oxidation [91]. There are other hydrophilic surface modifications that can be considered, including poly[N- (2-hydroxypropyl) methacrylamide] (poly(HPMA)) brush [92] or polysaccharides like heparin [93], hyaluronic acid [94] and chitosan [95,96]. Zwitterionic polymers can be alternatively used to confer hydrophilicity to surfaces. The most common zwitterionic polymers are phosphorylcholine, sulfobetaine and carboxybetaine [74,97,98]. Although they have been extensively studied as the next generation of promising antifouling materials [84], the long-term stability of zwitterionic surface is still a concern [74]. The use of amphiphilic polymeric coatings, by the combination of dodecyl methacrylate, polyethylene glycol methacrylate and acrylic acid, has also proven to prevent bacterial adhesion significantly [56]. An amphiphilic coating which was effective against both bacteria and protein adhesion was performed by synthesising a polymer from hydrophobic benzyl methacrylate, hydrophilic polyethylene glycol methacrylate and methacrylic acid [99]. Another amphiphilic moiety reported in the literature is the use of biosurfactants such as surfactin, rhamnolipids and several other surface active compounds produced by microorganisms [100]. Biosurfactants are advantageous due to their low toxicity, biodegradability and biocompatibility. Furthermore, they show both anti-adhesive and antimicrobial properties and thus they offer a great potential to be used in urinary catheters [101].
In general, thin hydrophilic coatings like polymer brushes may not be mechanically stable in the long-term use. Thus, hydrogel coatings can be promising considering biocompatibility, functional group density and lubricity. The problem with hydrogel coatings is that they are fragile due to weak interactions between the coating and the substrate. Nevertheless, crosslinking of the coating to the substrate can avoid this problem while improving its anti-adhesive performance [102]. Hydrogels can also be used in combination with antimicrobial agents. In a study performed by Su et al [103], a hydrogel from PEG oligomers modified with the antimicrobial agent methacrylate modified polyhexamethylene quinidine, showed potential use.
Although antifouling surfaces are advantageous against antimicrobial resistance, the results obtained by anti-adhesive surface modifications are generally considered to be modest compared to surfaces modified with antimicrobial agents [56]. Hence, modifications employing the combination of both strategies are being developed. Polyethylene glycol has been a popular choice in studies combining antifouling and antimicrobial functions. A study with antimicrobial and antifouling properties was done by combining polyethylene glycol with quaternary ammonium salts [104]. Another study with switchable surface features was also designed by combining polyethylene glycol as antifouling and poly[2-(dimethyl decyl ammonium)ethyl methacrylate] as bactericidal lower layer [105]. In a study done by Li et al [106], a coating consisting of copper ions incorporated in poly(3-sulfopropyl methacrylate potassium salt) brushes was developed. The study showed that the use of copper ions as antimicrobials increased the efficiency of antifouling surfaces. In another study, zwitterionic polymer poly(sulfobetaine methacrylate) as antifouling and quaternary ammonium salts as antimicrobial agents were combined and were found to effectively prevent biofilm formation [107]. Moreover, antimicrobial peptides were also combined with zwitterion poly(3-[dimethyl- [2-(2-methylprop-2-enoyloxy)ethyl] azaniumyl]propane-1-sulfonate), polymer brushes as an antifouling lower layer which provided significant inhibition in bacterial adhesion [108]. Furthermore, combining antifouling surfaces with antimicrobial surfaces can also increase life cycle of the surface modification as dead bacteria cells can be removed from the surface with antifouling functionality [106].
Additional to hydrophilic surface modifications, the development of superhydrophobic surfaces [109] is another anti-adhesive surface approach that can be explored to prevent and/ or reduce bacterial adhesion. The surfaces presenting water contact angles higher than 150° are called superhydrophobic surfaces. The anti-adhesive principle of superhydrophobic surfaces is the formation of solid-air-liquid, air pockets, interfaces within micro / nano morphologies. Thus, both surface chemical composition and roughness plays a role [89]. Although superhydrophobic surfaces can provide anti-adhesive features through formation of air pockets, they can be released from the surfaces over time, thus limiting the use of these type of surfaces [33,34]. Nevertheless, superhydrophobic surfaces still attract attention and their effectiveness has been evaluated through different methods and performance tests. In a recent study, trifluoropropyl was used for superhydrophobic surface modification and provided a reduced bacterial adhesion due to cleaning features [110]. Furthermore, both antimicrobial and antifouling strategy of superhydrophobic surfaces could be used to modify the surface. In a study by Zhang et al, [109], silver nanoparticles were modified with 1H,1H,2H,2H-perfluorodecanethiol, which provided anti-adhesive properties. Additionally, it was shown that non-stick polytetrafluoroethylene coating combined with silver inhibited bacterial adhesion [111,112].
Other Strategies
In addition to the use of antimicrobial and antifouling surfaces, several other approaches have been explored. There have been studies to reduce bacterial adhesion by forming specific micro and nano patterns on the surface topography. These surfaces reduce the bacterial adhesion by reducing the amount of accessible area for bacteria to adhere onto the surface [8,113]. Further modification via lubricant immersion on nanopatterned or porous surfaces can result in highly effective surfaces [114]. Furthermore, those nanopatterns can also be arranged in a way to provide bactericidal properties to the surface [115]. However, it is important to note that nanopattern design parameters like shape, height, diameter and spacing affect the anti-adhesive behaviour of the surface [33,116].
Some other approaches incorporate viral and bacterial treatment which can be alternative to antimicrobial compounds. For example, bacteriophage therapy [117], has provided encouraging results, although the drawback is the strain specific host range and lack of clear regulation [8,118]. Probiotic coatings as displacement of pathogenic bacteria on urinary catheters have also provided some success [119]. Probiotics work based on the competitive exclusion properties and specific bacterial adhesion ability. For example, lactic acid producing probiotics can have several benefits including antimicrobial, antiadhesive and anti-QS (quorum-sensing) signalling. They further reduce the pH of the environment and this could also mean anti-encrustation properties [120]. Signal interference is another strategy to hinder bacterial communication within biofilm [74]. A coating with acylase PvdQ (an acylase with an N-terminal nucleophile (Ntn-hydrolase) which is a part of the pyoverdine gene cluster (pvd)) reduces biofilm formation by inactivating long chain Nacyl homoserine lactones (AHL) in QS system [121].
Biodegradable catheter alternatives can be another option to prevent bacterial adhesion and encrustation. Although variable degradation or fragment formation are current problems [122], biodegradable stents with antimicrobials, such as peptides and copper ions, have shown potential in urinary applications [123]. This kind of constantly renewable antimicrobial surfaces could represent the solution to both bacterial adhesion and encrustation, if the problems with un-uniform degradation or fragment formation can be solved.
Conclusions & future perspectives
Urinary catheters are commonly used medical devices for which bacterial adhesion remains a huge health problem. Some of the most critical challenges that are involved in the bacterial adhesion phenomena are: dealing with complex body fluids and molecules; a significant high number of bacteria types; physicochemical interactions with the materials surfaces and problems resulting from encrustation. Understanding bacterial adhesion is key to develop strategies and design new anti-adhesive materials for catheters. Several approaches, including hydrophilic coatings, hydrogels, antimicrobial coatings and surface patterning or combinations thereof, seem to offer interesting results. However, despite all the progresses in the material science field, there is still a great need for durable materials that can efficiently deal with the abovementioned challenges. Achieving durable and antibacterial surfaces with only one strategy like antimicrobial or anti-adhesive is not an easy task as both strategies have disadvantages; thus, a proper combination of synergistic approaches can be beneficial at obtaining more resistant surfaces for a longer usage period. Moreover, probiotic coatings seem to be an interesting option for future applications, considering that they have a combination of several strategies like antimicrobial, antiadhesive, and anti-QS. However, the stability and shelf-life of such coatings might need to be considered. Another recently explored alternative is the use of biodegradable catheters made from hydrogels, being this a promising approach to develop anti-adhesive properties while preventing encrustation due to constant dissolving state, which warrants further research and development.
Acknowledgments
This work was supported by the ViBrANT project that received funding from the EU Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie, Grant agreement no 765042 and the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020.
References
- Muhammad MH, Idris AL, Fan X, Guo Y, Yu Y, et al. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front Microbiol. 2020; 11: 928.
- Adlhart C, Verran J, Azevedo NF, Olmez H, Keinänen-Toivola MM, et al. Surface modifications for antimicrobial effects in the healthcare setting: a critical overview, J. Hosp. Infect. 2018; 99: 239-249.
- Falde EJ, Yohe ST, Colson YL, Grinstaff MW. Superhydrophobic materials for biomedical applications. Biomaterials. 2016; 104: 87-103.
- Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, et al. Group, Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two european point prevalence surveys, 2016 to 2017, Eurosurveillance. 2018; 23: 1-17.
- Smith KF, Goldberg M, Rosenthal S, Carlson L, Chen J, et al. Global rise in human infectious disease outbreaks. J R Soc Interface. 2014; 11: 20140950.
- Zander ZK, Becker ML. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices, ACS Macro Lett. 2018; 7: 16-25.
- Zumstein V, Betschart P, Albrich WC, Buhmann MT, Ren Q, et al. Biofilm formation on uretral stents - incidence, clinical impact and prevention, Swiss Med Wkly. 2017; 147: w14408.
- Pelling H, Nzakizwanayo J, Milo S, Denham EL, MacFarlane WM, et al. Bacterial biofilm formation on indwelling urethral catheters, Lett. Appl. Microbiol. 2019; 68: 277-293.
- Cortese YJ, Wagner VE, Tierney M, Devine D. Fogarty, Review of catheter-associated urinary tract infections and in vitro urinary tract models. J Healthc Eng. 2018; 2986742.
- Berne C, Ellison CK, Ducret A, Brun YV. Bacterial adhesion at the single-cell level, Nat. Rev. Microbiol. 2018; 16: 616-627.
- Flemming H, Wingender J, Szewzyk U, Steinberg P, Rice SA, et al. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016; 14: 563-575.
- Kehinde EO, Rotimi VO, Al-hunayan A, Abdul-halim H, Boland F, et al. Bacteriology of Urinary Tract Infection Associated with Indwelling J Ureteral Stents. J Endourol. 2004; 18: 891-896.
- Ren Y, Wang C, Chen Z, Allan E, Van der Mei HC, et al. Emergent heterogeneous microenvironments in biofilms: substratum surface heterogeneity and bacterial adhesion force-sensing. FEMS Microbiol Rev. 2018; 42: 259-272.
- Ruan B, Wu P, Liu J, Jiang L, Wang H, et al. Adhesion of Sphingomonas sp. GY2B onto montmorillonite: A combination study by thermodynamics and the extended DLVO theory, Colloids Surfaces B Biointerfaces. 2020; 192: 111085.
- Dufrêne YF. Sticky microbes: forces in microbial cell adhesion, Trends Microbiol. 2015; 23: 376-382.
- Alam F, Kumar S, Varadarajan KM. Quantification of Adhesion Force of Bacteria on the Surface of Biomaterials: Techniques and Assays. ACS Biomater Sci Eng. 2019; 5: 2093-2110.
- Hori K, Matsumoto S. Bacterial adhesion: From mechanism to control. Biochem Eng J. 2010; 48: 424-434.
- Stickler DJ. Bacterial biofilms in patients with indwelling urinary catheters. Nat Clin Pract Urol. 2008; 5: 598-608.
- Ramstedt M, Ribeiro IAC, Bujdakova H, Mergulhão FJM, Jordao L, et al. Evaluating Efficacy of Antimicrobial and Antifouling Materials for Urinary Tract Medical Devices: Challenges and Recommendations, Macromol Biosci. 2019; 19: 1800384.
- Pajerski W, Ochonska D, Brzychczy-Wloch M, Indyka P, Jarosz M, et al. Attachment efficiency of gold nanoparticles by Gram-positive and Gram-negative bacterial strains governed by surface charges. J Nanoparticle Res. 2019; 21: 186.
- Ferraris S, Cochis A, Cazzola M, Tortello M, Scalia A, et al. Cytocompatible and Anti-bacterial Adhesion Nanotextured Titanium Oxide Layer on Titanium Surfaces for Dental and Orthopedic Implants. Front Bioeng Biotechnol. 2019; 7: 103.
- Poortinga AT, Bos R, Norde W, Busscher HJ. Electric double layer interactions in bacterial adhesion to surfaces, 2002.
- Gross M, Cramton SE, Götz F, Peschel A. Key Role of Teichoic Acid Net Charge in Staphylococcus aureus Colonization of Artificial Surfaces. Infect Immun. 2001; 69: 3423–3426.
- Berne C, Ducret A, Hardy GG, Brun YV. Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. Microb Spectr. 2015; 3.
- An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998; 43: 338-348.
- Cai L, Wu D, Xia J, Shi H, Kim H. Influence of physicochemical surface properties on the adhesion of bacteria onto four types of plastics. Sci Total Environ. 2019; 671: 1101-1107.
- Van Der Mei HC, Bos R, Busscher HJ. A reference guide to microbial cell surface hydrophobicity based on contact angles, Colloids Surfaces B Biointerfaces. 1998; 11: 213–221.
- Kim KW. Electron microscopic observations of prokaryotic surface appendages. J Microbiol. 2017; 55: 919-926.
- Gunardi WD, Karuniawati A, Umbas R, Bardosono S, Lydia A, et al. Biofilm-Producing Bacteria and Risk Factors (Gender and Duration of Catheterization) Characterized as Catheter-Associated Biofilm Formation. Int J Microbiol. 2021; 8869275.
- Szymanski CM, Schnaar RL, Aebi M. Bacterial and Viral Infections, in: Varki A, Cummings RD, Esko JD, Stanley P, G.W. Hart, M. Aebi, A.G. Darvill, T. Kinoshita, N.H. Packer, J.H. Prestegard, R.L. Schnaar, P.H. Seeberger (Eds.), Essentials Glycobiol., 3rd ed., Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. 2021; 1-10.
- Azeredo J, Visser J, Oliveira R. Exopolymers in bacterial adhesion : interpretation in terms of DLVO and XDLVO theories, Colloids Surfaces B Biointerfaces. 1999; 14: 141-148.
- Pakkulnan R, Anutrakunchai C, Kanthawong S, Taweechaisupapong S, Chareonsudjai P, et al. Extracellular DNA facilitates bacterial adhesion during Burkholderia pseudomallei biofilm formation. PLoS One. 2019; 14: 1-8.
- Çaykara T, Sande MG, Azoia N, Rodrigues LR, Silva CJ. Exploring the potential of polyethylene terephthalate in the design of antibacterial surfaces. Med Microbiol Immunol. 2020; 209: 363- 372.
- Zhang X, Wang L, Levänen E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013; 3: 12003–12020.
- Maikranz E, Spengler C, Thewes N, Thewes A, Nolle F, et al. Different binding mechanisms of: Staphylococcus aureus to hydrophobic and hydrophilic surfaces. Nanoscale. 2020; 12: 19267- 19275.
- Maan AMC, Hofman AH, De Vos WM, Kamperman M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Advenced Funct Mater. 2020; 30: 2000936.
- Zhao Q, Wang S, Müller-Steinhagen H. Tailored surface free energy of membrane diffusers to minimize microbial adhesion. Appl Surf Sci. 2004; 230: 371-378.
- Liu Y, Zhao Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys Chem. 2005; 117: 39-45.
- Katsikogianni M, Amanatides E, Mataras D, Missirlis YF. Staphylococcus epidermidis adhesion to He, He/O2 plasma treated PET films and aged materials: Contributions of surface free energy and shear rate, Colloids Surfaces B Biointerfaces. 2008; 65: 257-268.
- van Loosdrecht MCM, Zehnder AJB. Energetics of bacterial adhesion, Experientia. 1990; 46: 817-822.
- Wu S, Zhang B, Liu Y, Suo X, Li H. Influence of surface topography on bacterial adhesion: A review (Review). Biointerphases. 2018; 13: 060801.
- Wu S, Altenried S, Zogg A, Zuber F, Maniura-Weber K, et al. Role of the Surface Nanoscale Roughness of Stainless Steel on Bacterial Adhesion and Microcolony Formation. ACS Omega. 2018; 3: 6456–6464.
- Friedlander RS, Vlamakis H, Kim P, Khan M, Kolter R, et al. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc Natl Acad Sci. 2013; 110: 5624-5629.
- Quéré D. Rough ideas on wetting. Phys A Stat Mech.Its Appl. 2002; 313: 32-46.
- Cassie ABD, Baxter S. Wettability of porous surfaces, Trans. Faraday Soc. 1944; 40: 546-551.
- Perera-Costa D, Bruque JM, González-Martín ML, Gómez-García AC, Vadillo-Rodríguez V. Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir. 2014; 30: 4633-4641.
- Echeverria C, Torres MDT, Fernández-García M, de la FuenteNunez C, Muñoz-Bonilla A. Physical methods for controlling bacterial colonization on polymer surfaces. Biotechnol Adv. 2020; 43: 107586.
- Elbourne A, Chapman J, Gelmi A, Cozzolino D, Crawford RJ, et al. Bacterial-nanostructure interactions: The role of cell elasticity and adhesion forces. J Colloid Interface Sci. 2019; 546: 192-210.
- Straub H, Bigger CM, Valentin J, Abt D, Qin XH, et al. Bacterial Adhesion on Soft Materials: Passive Physicochemical Interactions or Active Bacterial Mechanosensing? Adv Healthc Mater. 2019; 8: 1801323.
- Kolewe KW, Zhu J, Mako NR, Nonnenmann SS, Schiffman JD. Bacterial Adhesion is Affected by the Thickness and Stiffness of Poly(ethylene glycol) Hydrogels, ACS Appl. Mater. Interfaces. 2018; 10: 2275-2281.
- Rose C, Parker A, Jefferson B, Cartmell E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit Rev Environ Sci Technol. 2015; 45: 1827-1879.
- Katsikogianni M, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cells Mater. 2004; 8: 37-57.
- Baumann JM, Affolter B. From crytalluria to kidney stones, some physicochemical aspects of calcium nephrolithiasis. World J Nephrol. 2014; 3: 2 56–267.
- Raman N, Lee MR, de L A. Rodríguez López, Palecek SP, Lynn DM. Antifungal activity of a b -peptide in synthetic urine media: Toward materials-based approaches to reducing catheter-associated urinary tract fungal infections. Acta Biomater. 2016; 43: 240-250.
- Amalaradjou MAR, Venkitanarayanan K. Role of Bacterial Biofilms in Catheter-Associated Urinary Tract Infections (CAUTI) and Strategies for Their Control, in: T. Nelius (Ed.). Recent Adv Urin Tract Infect Intech Open. 2013.
- Keum H, Kim JY, Yu B, Yu SJ, Kim J, et al. Prevention of bacterial colonization on catheters by a one-step coating process involving an antibiofouling polymer in water. ACS Appl Mater Interfaces. 2017; 9: 19736-19745.
- Tenke P, Kovacs B, Jäckel M, Nagy E. The role of biofilm infection in urology. World J Urol. 2006; 24: 13-20.
- Han A, Tsoi JKH, Rodrigues FP, Leprince JG, Palin WM. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int J Adhes Adhes. 2016; 69: 58-71.
- Martín ML, Pfaffen V, Valenti LE, Giacomelli CE. Albumin biofunctionalization to minimize the Staphylococcus aureus adhesion on solid substrates, Colloids Surfaces B Biointerfaces. 2018; 167: 156-164.
- Sinha SD, Chatterjee S, Maiti PK, Tarafdar S, Moulik SP. Evaluation of the role of substrate and albumin on Pseudomonas aeruginosa biofilm morphology through FESEM and FTIR studies on polymeric biomaterials. Prog Biomater. 2017; 6: 27-38.
- He T, Shi ZL, Fang N, Neoh KG, Kang ET, Chan V. The effect of adhesive ligands on bacterial and fibroblast adhesions to surfaces, Biomaterials. 2009; 30: 317-326.
- Li C, Ding Y, Kuddannaya S, Zhang Y, Yang L. Anti-bacterial properties of collagen-coated glass and polydimethylsiloxane substrates. J Mater Sci. 2017; 52: 9963-9978.
- Gabi M, Hefermehl L, Lukic D, Zahn R, Vörös J, et al. Electrical microcurrent to prevent conditioning film and bacterial adhesion to urological stents. Urol Res. 2011; 39: 81-88.
- Trautner BW, Darouiche RO. Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control. 2004; 32: 177-183.
- Denstedt JD, Wollin TA, Reid G, Ph D. Biomaterials Used in Urology: Current Issues of Biocompatibility, Infection, and Encrusta tion. J Of Endourology. 1998; 12: 493-500.
- Stickler DJ, Morgan SD. Observations on the development of the crystalline bacterial biofilms that encrust and block Foley catheters. J Hosp Infect. 2008; 69: 350-360.
- Stickler DJ, Feneley RCL. The encrustation and blockage of longterm indwelling bladder catheters: A way forward in prevention and control, Spinal Cord. 2010; 48: 784-790.
- Ferreira M, Rzhepishevska O, Grenho L, Malheiros D, Gonçalves L, et al. Bettencourt, Levofloxacin-loaded bone cement delivery system: highly effective against intracellular bacteria and Staphylococcus aureus biofilms. Int J Pharm. 2017; 532: 241-248.
- Sjollema J, Zaat SAJ, Fontaine V, Ramstedt M, Luginbuehl R, et al. In vitro methods for the evaluation of antimicrobial surface designs. Acta Biomater. 2018; 70: 12-24.
- Azevedo AS, Almeida C, Gomes LC, Ferreira C, Mergulhão FJ, et al. An in vitro model of catheter-associated urinary tract infections to investigate the role of uncommon bacteria on the Escherichia coli microbial consortium. Biochem Eng J. 2017; 118: 64-69.
- Chua RYR, Lim K, Leong SSJ, Tambyah PA, Ho B. An in-vitro urinary catheterization model that approximates clinical conditions for evaluation of innovations to prevent catheter-associated urinary tract infection. J Hosp Infect. 2017; 97: 66-73.
- Buhmann MT, Stiefel P, Maniura-Weber K, Ren Q. In Vitro Biofilm Models for Device-Related Infections. Trends Biotechnol. 2016; 34: 945-948.
- Norsworthy AN, Pearson MM. From Catheter to Kidney Stone: The Uropathogenic Lifestyle of Proteus mirabilis. Trends Microbiol. 2017; 25: 304-315.
- Zhu Z, Wang Z, Li S, Yuan X. Antimicrobial strategies for urinary catheters. J Biomed Mater Res Part A. 2019; 107: 445-467.
- Singha P, Locklin J, Handa H. A Review of the Recent Advances in Antimicrobial Coatings for Urinary Catheters. Acta Biomater. 2017; 50: 20-40.
- Andersen MJ, Flores-Mireles AL. Urinary catheter coating modifications: The race against catheter-associated infections. Coatings. 2020; 10: 23.
- Wu K, Yang Y, Zhang Y, Deng J, Lin C. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int J Nanomedicine. 2015; 10: 7241-7252.
- Mala R, Aglin AA, Celsia ASR, Geerthika S, Kiruthika N, et al. Foley catheters functionalised with a synergistic combination of antibiotics and silver nanoparticles resist biofilm formation. IET Nanobiotechnology. 2017; 11: 612-620.
- Homeyer KH, Goudie MJ, Singha P, Handa H. Liquid-Infused Nitric-Oxide-Releasing Silicone Foley Urinary Catheters for Prevention of Catheter-Associated Urinary Tract Infections. ACS Biomater Sci Eng. 2019; 5: 2021-2029.
- Margel D, Mizrahi M, Regev-Shoshani G, Ko M, Moshe M, et al. Nitric oxide charged catheters as a potential strategy for prevention of hospital acquired infections. PLoS One. 2017; 12: e0174443.
- Thallinger B, Brandauer M, Burger P, Sygmund C, Ludwig R, et al. Cellobiose dehydrogenase functionalized urinary catheter as novel antibiofilm system. J Biomed Res Part B. 2016; 104: 1448-1456.
- Tran C, Yasir M, Dutta D, Eswaramoorthy N, Suchowerska N, et al Single Step Plasma Process for Covalent Binding of Antimicrobial Peptides on Catheters to Suppress Bacterial Adhesion, ACS Appl. Bio Mater. 2019; 2: 5739-5748.
- Mahlapuu M, Björn C, Ekblom J. Antimicrobial peptides as therapeutic agents: opportunities and challenges. Crit Rev Biotechnol. 2020; 40: 978-992.
- Nikam SP, Chen P, Nettleton K, Hsu YH, Becker ML. Zwitterion Surface-Functionalized Thermoplastic Polyurethane for Antifouling Catheter Applications. Biomacromolecules. 2020; 21: 2714-2725.
- Torzewska A, Rozalski A. Inhibition of crystallization caused by Proteus mirabilis during the development of infectious urolithiasis by various phenolic substances. Microbiol Res. 2014; 169: 579-584.
- Rubini D, Hari BNV, Nithyanand P. Chitosan coated catheters alleviates mixed species biofilms of Staphylococcus epidermidis and Candida albicans. Carbohydr Polym. 2021; 252: 117192.
- Bruenke J, Roschke I, Agarwal S, Riemann T, Greiner A. Quantitative Comparison of the Antimicrobial Efficiency of Leaching versus Nonleaching Polymer Materials. Macromol Biosci. 2016; 16: 647-654.
- Al Meslmani BM, Mahmoud GF, Leichtweiß T, Strehlow B, Sommer FO, et al. Covalent immobilization of lysozyme onto woven and knitted crimped polyethylene terephthalate grafts to minimize the adhesion of broad spectrum pathogens. Mater Sci Eng C. 2016; 58: 78-87.
- Liu L, Shi H, Yu H, Yan S, Luan S. The recent advances in surface antibacterial strategies for biomedical catheters. Biomater Sci. 2020; 8: 4095-4108.
- Park KD, Kim YS, Han DK, Kim YH, Lee EHB, et al. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials. 1998; 19: 851-859.
- Hucknall A, Rangarajan S, Chilkoti A. In Pursuit of Zero: Polymer Brushes that Resist the Adsorption of Proteins. Adv Mater. 2009; 21: 2441-2446.
- Alves P, Gomes LC, Vorobii M, Rodriguez-Emmenegger C, Mergulhão FJ. The potential advantages of using a poly(HPMA) brush in urinary catheters: effects on biofilm cells and architecture. Colloids Surfaces B Biointerfaces. 2020; 191: 110976.
- Tenke P, Riedl CR, Jones GL, Williams GJ, Stickler D, et al. Bacterial biofilm formation on urologic devices and heparin coating as preventive strategy. Int J Antimicrob Agents. 2004; 23: S67-74.
- Mohan T, Čas A, Bračic M, Plohl O, Vesel A, et al. Highly Protein Repellent and Antiadhesive Polysaccharide Biomaterial Coating for Urinary Catheter Applications. ACS Biomater Sci Eng. 2019; 5: 5825-5832.
- Rubini D, Vishnu P, Jayasankari S. Suppressing the phenotypic virulence factors of Uropathogenic Escherichia coli using marine polysaccharide. Microb Pthogenes. 2020; 141: 103973.
- Campana R, Biondo F, Mastrotto F, Baffone W, Casettari L. Chitosans as new tools against biofilms formation on the surface of silicone urinary catheters. Int J Biol Macromol. 2018; 118: 2193-2200.
- Diaz Blanco C, Ortner A, Dimitrov R, Navarro A, et al. Building an antifouling zwitterionic coating on urinary catheters using an enzymatically triggered bottom-up approach. ACS Appl Mater Interfaces. 2014; 6: 11385-11393.
- Peng W, Liu P, Zhang X, Peng J, Gu Y, et al. Multi-functional zwitterionic coating for silicone-based biomedical devices. Chem Eng J. 2020; 398: 125663.
- Woo J, Seo H, Na Y, Choi S, Kim S, et al. Facile synthesis and coating of aqueous antifouling polymers for inhibiting pathogenic bacterial adhesion on medical devices. Prog Org Coatings. 2020; 147: 105772.
- Rodrigues LR. Inhibition of bacterial adhesion on medical devices. Adv Exp Med Biol. 2011; 351-367.
- Zhang S, Liang X, Gadd GM, Zhao Q. Marine microbial-derived antibiotics and biosurfactants as potential new agents against catheter-associated urinary tract infections. Mar Drugs. 2021; 19: 255.
- Yong Y, Qiao M, Chiu A, Fuchs S, Liu Q, et al. Conformal Hydrogel Coatings on Catheters to Reduce Biofouling. Langmuir. 2019; 35: 1927-1934.
- Su Y, Feng T, Feng W, Pei Y, Li Z, et al. Mussel-Inspired, SurfaceAttachable Initiator for Grafting of Antimicrobial and Antifouling Hydrogels, Macromol. Rapid Commun. 2019; 40: 1-8.
- Li Z, Yang X, Liu H, Yang X, Shan Y, et al. Dual-functional antimicrobial coating based on a quaternary ammonium salt from rosin acid with in vitro and in vivo antimicrobial and antifouling properties. Chem Eng J. 2019; 374: 564-575.
- Zhang Y, Zhang X, Zhao YQ, Zhang XY, Ding X, et al. Self-adaptive antibacterial surfaces with bacterium-triggered antifouling-bactericidal switching properties. Biomater Sci. 2020; 8: 997-1006.
- Li P, Ding Z, Yin Y, Yu X, Yuan Y, et al. Cu2+-doping of polyanionic brushes: A facile route to prepare implant coatings with both antifouling and antibacterial properties. Eur Polym J. 2020; 134: 109845.
- He Y, Wan X, Xiao K, Lin W, Li J, et al. Anti-biofilm surfaces from mixed dopamine-modified polymer brushes: Synergistic role of cationic and zwitterionic chains to resist: Staphyloccocus aureus. Biomater Sci. 2019; 7: 5369-5382.
- Zhang XY, Zhao YQ, Zhang Y, Wang A, Ding X, et al. Antimicrobial Peptide-Conjugated Hierarchical Antifouling Polymer Brushes for Functionalized Catheter Surfaces. Biomacromolecules. 2019; 20: 4171-4179.
- Zhang S, Liang X, Zhao Q. Superhydrophobic Coatings for Urinary Catheters To Delay Bacterial Biofilm Formation and Catheter-Associated Urinary Tract Infection. Appl Bio Mater. 2020; 3: 282-291.
- Gayani B, Dilhari A, Kottegoda N, Ratnaweera DR, Weerasekera MM. Reduced Crystalline Biofilm Formation on Superhydrophobic Silicone Urinary Catheter Materials. ACS Omega. 2021; 6: 11488-11496.
- Wang L, Zhang S, Keatch R, Corner G, Nabi G, et al. In-vitro antibacterial and anti-encrustation performance of silver-polytetrafluoroethylene nanocomposite coated urinary catheters. J Hosp Infect. 2019; 103: 55-63.
- Zhang S, Wang L, Liang X, Vorstius J, Keatch R, et al. Enhanced Antibacterial and Antiadhesive Activities of Silver-PTFE Nanocomposite Coating for Urinary Catheters. ACS Biomater Sci Eng. 2019; 5: 2804-2814.
- Vasudevan R, Kennedy AJ, Merritt M, Crocker FH, Baney RH. Microscale patterned surfaces reduce bacterial fouling-microscopic and theoretical analysis. Colloids Surfaces B Biointerfaces. 2014; 117: 225-232.
- Epstein AK, Wong TS, Belisle RA, Boggs EM, Aizenberg J. Liquidinfused structured surfaces with exceptional anti-biofouling performance, Proc Natl Acad Sci U S A. 2012; 109: 13182-13187.
- Fontelo R, Soares da Costa D, Reis RL, Novoa-Carballal R, Pashkuleva I. Bactericidal nanopatterns generated by block copolymer self-assembly. Acta Biomater. 2020; 112: 174-181.
- Modaresifar K, Azizian S, Ganjian M, Fratila-Apachitei LE, Zadpoor AA. Bactericidal effects of nanopatterns: A systematic review. Acta Biomater. 2019; 83: 29-36.
- Milo S, Hathaway H, Nzakizwanayo J, Alves DR, Esteban PP, et al. Prevention of encrustation and blockage of urinary catheters by Proteus mirabilis via pH-triggered release of bacteriophage. J Mater Chem B. 2017; 5: 5403-5411.
- Furfaro LL, Payne MS, Chang BJ. Bacteriophage Therapy : Clinical Trials and Regulatory Hurdles. Front Cell Infect Microbiol. 2018; 8: 376.
- Chen Q, Zhu Z, Wang J, Lopez AI, Li S, et al. Probiotic E. coli Nissle 1917 biofilms on silicone substrates for bacterial interference against pathogen colonization. Acta Biomater. 2017; 50: 353-360.
- Khan F, Tabassum N, Kim YM. A strategy to control colonization of pathogens: embedding of lactic acid bacteria on the surface of urinary catheter, Appl. Microbiol. Biotechnol. 2020; 104: 9053-9066.
- Vogel J, Wakker-Havinga M, Setroikromo R, Quax WJ. Immobilized Acylase PvdQ Reduces Pseudomonas aeruginosa Biofilm Formation on PDMS Silicone. Front Chem. 2020; 8: 54.
- Barros AA, Oliveira C, Ribeiro AJ, Autorino R, Reis RL, et al. In vivo assessment of a novel biodegradable ureteral stent. World J Urol. 2018; 36: 277-283.
- Gao L, Wang Y, Li Y, Xu M, Sun G, et al. Biomimetic biodegradable Ag@Au nanoparticle-embedded ureteral stent with a constantly renewable contact-killing antimicrobial surface and antibiofilm and extraction-free properties, Acta Biomater. 2020; 114: 117-132.