Research article
Volume 2, Issue 4
Cytotoxic and Antimicrobial Efficacy of Alhagi maurorum
Sobia Gilani; Yamin Bibi*; Tayyiba Afzal; Rimsha Baloch
Department of Botany, Pir Mehr Ali Shah Arid Agriculture University Rawalpindi Islamabad, Punjab, Pakistan.
Corresponding Author :
Yamin Bibi
Email: dryaminbibi@uaar.edu.pk
Received : Mar 10, 2023 Accepted : Apr 20, 2023 Published : Apr 27, 2023 Archived : www.meddiscoveries.org
Citation: Gilani S, Bibi Y, Afzal T, Baloch R. Cytotoxic and Antimicrobial Efficacy of Alhagi maurorum. Med Discoveries. 2023; 2(4): 1036.
Copyright: © 2023 Bibi Y. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Medicinal plants have long been studied due to their anticancer effects and use of them is commonly increased as a complementary and alternative medicine among patients with cancer. As drugs obtained from plants are cheap, safe to use, available without any difficulty, more effective, easy to store, and rarely have side effects. Alhagi maurorum, a member of family Fabaceae contained many secondary metabolites and used in folk medicines as laxative, purgative, diaphoretic, expectorant and diuretic. Extracts were prepared by cold maceration technique. Different extracts (Crude, Methanol, Ethyl acetate and n-Hexane) of A. maurorum from aerial part were screened for Total Phenolic, and Tannins content, Antibacterial, Antifungal and Cytotoxic activities. N-hexane extract showed high TTC, TAC and TPC content. Cytotoxic properties were examined by Brine Shrimp Lethality Assay. Different concentrations (1000, 750, 500, 250, 100 and 50 µg/mL) of Methanol, Ethyl acetate and n-Hexane extract were tested against 10 nauplii. Cytotoxic activity was dose dependent. LD50 of following extracts was 381.40 ppm, 160.27 ppm and 385.72ppm respectively. The antimicrobial activity was determined against 4 pathogenic bacterial strains (Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Pseudomonas sp.) using Agar Well Diffusion Method. All extracts showed concentration dependent inhibition against tested strains. The maximum Zone of Inhibition was observed by Crude (11.3 ± 1.1, 10.3 ± 0.5) and ethyl acetate (10.3 ± 0.5, 10 ± 1.0) at 100 µg/ml against Staphylococcus aureus, Staphylococcus epidermidis respectively. Antifungal action of Methanol and n-Hexane extracts (10, 25, 50 µg/ml) were examined by Agar Tube Dilution method against Fusarium and Alternaria sp. At 50 µg/ml highest percentage inhibition was observed by Methanol extract for both the strains i.e. 91.8% for Alternaria sp. and 80% for Fusarium sp. The present study revealed that A. maurorum carries powerful antimicrobial and remarkable cytotoxic activities, could be a potential source of antibiotics and anticancer compounds. This plant ought to be considered for additional phytochemical examination and pharmacological assessment.
Keywords: Phytochemicals; Cytotoxic; Antimicrobial; Alhagi maurorum.
Introduction
Plants have been used as traditional medicine since ancient times, and they are the source of many modern pharmaceutical medicines [1]. The interest in using natural products as medicines, has been driven by the Exploring methods for collecting the necessary plant materials for pharmacological screening and drug development [2]. As drugs made from plants are generally less expensive, safer to use, more accessible, more effective, and easier to store [3].
Alhagi maurorum, a member of family Fabaceae, commonly known as Camelthorn bush or manna, grows in various places in the world such as Asia, Europe and North Africa [4]. Plant has long been used in folk medicine due to its richness in pharmacologically active metabolites [5]. The whole plant of A. maurorum has laxative, purgative, diaphoretic, expectorant and diuretic [5]. It is traditionally used by the local communities against rheumatoid, liver disorders, infections of urinary tract, stomach and intestinal disorders [6].
Material and methodology
Collection and extraction
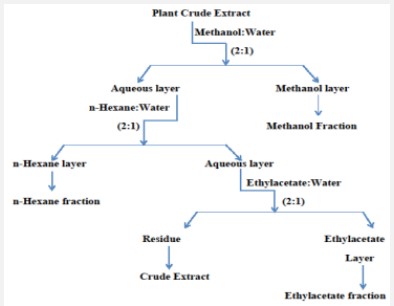
Whole-plant of Alhagi maurorum was collected from DI,Khan during summer season. Material was shade dried and ground into fine powder. Powdered sample was stored for further experimentation.
Crude extract was prepared by the technique of cold maceration. The Crude extract was fractioned into, n-Hexane, ethyl acetate and methanolic extract [7].
Phytochemical screening
Phytochemical screening was done by few modifications in the methods used by [8].
Qualitative-analysis of sample was done to find out the presence of resins, saponins, alkaloids, flavonoids, phenolics, cardiac-glycosides, tannins, terpenoids, coumarins, phlobatannis, protein, carbohydrates and steroids.
Phytochemical screening demonstrated the presence of TPC, TAC and TTC was determined by FC method.
Cytotoxicity test (Brine shrimp lethality assay)
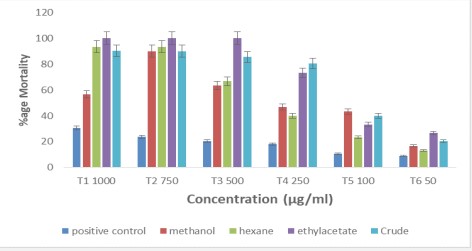
Cytotoxicity was tested by Brine shrimp lethality assay given by [9]. Sea salt (39 g) was mixed in 1 g of distilled water to make salt water. Ten nauplii were transferred to each test tube, containing artificial sea water. Different concentrations (100, 750, 500, 250, 100 and 50 µg/ml) of prepared fractions (n-Hexane, Methanol and ethyl-acetate) were added to each test tube. Incubated at 27oC for 24 hrs. The percentage mortality will be collected by the formula:
Death percentage =
IC50 value will calculated by linear regression equation that was obtained by plotting concentrations against cytotoxic activity.
Antimicrobial activities
Antibacterial activity
Antibacterial efficacy of fractions was tested by Agar well diffusion method proposed by [10]. Three different concentrations of crude methanol, n-hexane and ethyl-acetate extracts were prepared (100, 75 and 50 µg/ml). Two Gram positive (Staphylococcus epidermidis and Staphylococcus aureus) and two Gram negative, bacterial strains (Escherichia coli and Pseudomonas sp.) were used.
The % age growth inhibition was calculated with reference to the standard drug, according to the formula:
Percentage inhibition= (X-Y)/(Z-Y) × 100.
Antifungal activity
Antifungal activities of Alhagi maurorum, was tested by Agar tube dilution ,method by [11]. Different concentrations of methanol and n-hexane extracts were prepared (50, 25 and 10 µg/ml). Two different Pathogenic fungal, strains were used (Alternaria and Fusarium). Inoculum was added in each tube and left for one day at 28°C. The growth inhibition was calculated by given formula:
% age Inhibition= Growth in test-extract (mm) ÷ Growth in control (mm) × 100
Results and discussion
Cytotoxicity test
Ethyl acetate extract showed maximum mortality as compared to other extracts, with LD50 value of 160.27 ppm. Methanol extract showed maximum death rate at 750 and 500 µg/ ml with LD50 value of 381.40 ppm. While in case of n-Hexane maximum death was observed at high concentrations i.e. 1000 and 750 µg/ml with highest LD50 value of 385.72 ppm. Hence concluded that ethyl acetate was more toxic than methanol and n-Hexane extracts. Our results indicates that cytotoxicity is concentration dependent. Same outcomes were achieved by [12] examined Brine shrimp lethality assay of Spilanthes paniculata plant extracts.
Antimicrobial activity
Antibacterial activity
Ethyl acetate showed maximum inhibition against all the bacterial strains. Experiment showed that S. aureus showed maximum Z.O.I against all the extracts. Among all bacterial strains, E.coli is least susceptible to all the extracts and showed highest Z.O.I value 4.4 mm in ethyl acetate extract and S. aureus to be most vulnerable towards all extracts and showed highest Z.O.I value 11.3mm in ethyl acetate extract. These are according to the findings of [13], reported that gram –ive bacteria E.coli to be least susceptible and S. aureus showed maximum inhibition against all extracts.
All extracts showed concentration dependent inhibition against tested strains. As higher the concentration results into maximum inhibition zone [14] also observed concentration dependence of all extracts. Antimicrobial action are due to presence of antimicrobial constituents in plants i.e. Steroids, Alkaloids, Phenolic, flavonoids etc. [15]. If Alhagi maurorum is subjected to further studies would lead to the isolation of more bioactive compounds [16].
Antifungal activity
It is cleared from results of Antifungal assay that highest percentage inhibition was observed against Fusarium and Alternaria sp. by methanol extract as compared to n-Hexane. Percentage inhibition increased with increase in concentration (10, 25 and 50 µg/ml). At 50 µg/ml highest percentage inhibition was observed by Methanol extract for both the strains i.e. 91.8% and 80% for Alternaria and Fusarium sp. respectively. Methanol extract was tested against 14 fungal strains and found it to be effective against fungal strains, reported by [17]. In conclusion Fusarium sp. was found more susceptible against extracts of selected plant as compared to Alternaria sp. and methanol extract showed more antifungal activity than n-hexane as mentioned in the Table 2.
Phytochemical analysis
Qualitative analysis
Phytochemicals are tested against four different extracts (Crude, Methanol, n-Hexane and ethyl acetate extracts). The outcome of chemicals of plant determined of all fractions and crude extract were summarized in Table 3.
Table 1: Bacterial inhibition (In % age) of Different extracts of Alhagi maurorum.
| Zone of Inhibition (mm) (Mean ± S.D) | |||||
|---|---|---|---|---|---|
| Extract | Conc. | Gram(+) | Gram(-) | ||
| Staphylococcus aureus | taphylococcus epidermidis | Escherichia coli | Pseudomonas sp. | ||
| Crude | 100 µg/ml | 10.3 ± 0.5 | 10.3 ± 0.5 | 3.5 ± 0.7 | 6.8 ± 2.2 |
| 75 µg/ml | 10 ± 2.0 | 9.2 ± 0.8 | 2.4 ± 0.3 | 4.1 ± 0.1 | |
| 50 µg/ml | 4.4 ± 1.5 | 4.7 ± 1.6 | - | 0.6 ± 1.1 | |
| n-Hexane | 100 µg/ml | 8.3 ± 2.8 | 9 ± 0 | 4 ± 1.4 | 3.4 ± 1.5 |
| 75 µg/ml | 6 ± 3 | 5.3 ± 1.5 | 0.7 ± 1.0 | 4.3 ± 2.5 | |
| 50 µg/ml | 3.4 ± 2.2 | 4.4 ± 1.5 | - | 1.6 ± 1.5 | |
| Ethyl acetate | 100 µg/ml | 11.3 ± 1.1 | 10.74 ± 1.0 | 4.4 ± 0.8 | 7.0 ± 0.6 |
| 75 µg/ml | 9.6 ± 0.5 | 7 ± 1.0 | 2 ± 0 | 3.3 ± 1.5 | |
| 50 µg/ml | 3.4 ± 1.4 | 3.1 ± 0.7 | - | 0.6 ± 1.1 | |
| Methanol | 100 µg/ml | 7.74 ± 2.8 | 9.50 ± 2.5 | 3.1 ± 1.1 | 7.5 ± 1.8 |
| 75 µg/ml | 5.10 ± 1.9 | 6.6 ± 1.09 | 1.15 ± 0.5 | 5.65 ± 1.3 | |
| 50 µg/ml | 3.21 ± 1.5 | 3.35 ± 4.4 | - | 2.1 ± 1.2 | |
| Aqueous | 100 µg/ml | 3.3 ± 1.5 | 1.3 ± 2.3 | 2.4 ± 0.4 | - |
| 75 µg/ml | 2 ± 0.1 | 0.6 ± 1.1 | - | - | |
| 50 µg/ml | - | - | - | - | |
| Cefotaxime | 100 µg/ml | 14.5 ± 2.1 | 15.3 ± 1.1 | 7.2 ± 2.3 | 8.4 ± 0.7 |
| 75 µg/ml | 12.3 ± 3.5 | 10 ± 5.0 | 5.6 ± 2.0 | 5 ± 1.0 | |
| 50 µg/ml | 8.4 ± 1.0 | 6.6 ± 2.8 | 3.3 ± 1.2 | 3.3 ± 2.3 | |
Table 2: Fungal inhibition (Percentage inhibition ± S.D) of Methanol and n-Hexane extracts of Alhagi maurorum.
| Extracts | Concentration | Fusarium sp. | Alternaria sp. |
|---|---|---|---|
| Crude | 50 µg/ml | 61 ± 1.09 | 65.54 ± 1.2 |
| 25 | 34.4 ± 2.0 | 41 ± 2.2 | |
| 10 | 13.23 ± 1.1 | 19 ± 1.9 | |
| Ethyl acetate | 50 µg/ml | 72.2 ± 1.50 | 79.90 ± 2.0 |
| 25 | 66.15 ± 2.1 | 70.10 ± 1.2 | |
| 10 | 35 ± 1.0 | 51.09 ± 2.7 | |
| n-Hexane | 50 µg/ml | 65.2 ± 1.04 | 74.22 ± 2.20 |
| 25 | 47.09 ± 1.98 | 68 ± 1 | |
| 10 | 22.27 ± 2.08 | 61.15 ± 0.2 | |
| Methanol | 50 µg/ml | 80 ± 5.77 | 91.8 ± 5.03 |
| 25 | 73.30 ± 2.10 | 88.4 ± 4.5 | |
| 10 | 44 ± 1.73 | 79.56 ± 2.8 | |
| DMSO | 50 µg/ml | 21.23 ± 3.1 | 21.06 ± 1.2 |
| 25 | - | 5.4 ± 1.0 | |
| 10 | - | - | |
| Fluconazole | 50 µg/ml | 89.2 ± 1.8 | 92.2 ± 3.2 |
| 25 | 80.4 ± 1.0 | 79.5 ± 1.6 | |
| 10 | 65.9 ± 2.0 | 50.05 ± 0.2 |
Table 3: Qualitative Phytochemical analysis of Alhagi maurorum.
| Phytoconstituents | Observation | Crude extract | Methanol extract | n-Hexane extract | Ethyl-acetate Extract |
|---|---|---|---|---|---|
| Resin | Turbidity | + | + | + | + |
| Saponins | Forth formation | + | + | - | + |
| Tannin | Appearance of blackish/ brownish green/blue color | + | + | + | + |
| Phenolics | Blue color | + | + | + | + |
| Terpenoids | Reddish brown color | + | + | + | + |
| Alkaloids | Yellow/brown precipitates | + | + | + | + |
| Flavonoids | Dark yellow color | + | - | - | + |
| Cardiac glycosides | Brown ring | + | + | + | + |
| Coumarins | Yellow fluorescence | + | + | - | + |
| Phlobatannins | Red color precipitates | + | + | + | - |
| Steroids | Blue-Green ring | + | + | + | + |
| Proteins | White precipitates | + | + | - | + |
| Carbohydrates | Red/dull violet color | + | + | + | + |
(+) = Presence, (-) = Absent.
Table 4: Quantitative phytochemical analysis of various extracts of Alhagi maurorum.
| Quantitative Phytochemica | Methanol | Ethyl acetate | n-Hexane |
|---|---|---|---|
| Total Phenolic content | 59.7 ± 4.0 | 76.8 ± 1.8 | 50.3 ± 0.26 |
| Total Tannins content | 2.01 ± 0.05 | 4.52 ± 0.09 | 1.55 ± 0.65 |
| Total Alkaloid content | 32.2 ± 1.5 | 54 ± 0.22 | 21 ± 0.55 |
Quantitative analysis
Alhagi maurorum was analyzed for quantitative phytochemicals characterization for three major groups of phytochemicals which are Total phenolic, alkaloids and tannins content in plants. All the selected extracts showed high TTC, TAC and TPC and results have been given in Table 4.
TTC, TPC and TAC were found high in ethyl acetate extract. All extracts of Alhagi maurorum showed high TPC presence than TAC and TTC. The TPC of three extracts ranged 50.3±0.26 to 76.8 ± 1.8 (mg/gm). The TTC and TAC were also found high in ethyl acetate extract with the values 4.52 ± 0.09 and 54 ± 0.22 respectively.
Conclusion and recommendations
This work is the foremost on the biological action of different extracts (methanol, n-Hexane and ethyl-acetate) of Alhagi maurorum w.r.t cytotoxicity. Pharmacological evaluation of this plant showed great antimicrobial and cytotoxic potential. Plant can be recommended as selective fungicide, as it showed inhibitory effect against fungal strains (Fusarium and Alternaria sp). Alhagi maurorum contain some important bioactive compounds responsible for therapeutic activities. This plant may serve as an important source of medication. These results could be serving as benchmark for next studies. A very detailed study is required to find out the mechanism behind these effects. It is suggested that medicinal components of various parts of plant should be isolated in future studies.
Declarations
Author contributions
Yamin Bibi & Sobia Gilani: conceptualization, investigation, and writing original draft. Tayyiba Afzal & Rimsha Baloch: Proofreading. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Data availability statement: The data presented in this study are available upon fair request from the corresponding authors.
Acknowledgments: The authors would like to thanks Dr. Yamin Bibi, Arid Agriculture University Rawalpindi, for providing all the possible guidelines and equipment to carry out this research.
Conflicts of interest: No conflicts of interest.
References
- Salmerón-Manzano E, Garrido-Cardenas JA, Manzano-Agugliaro F. Worldwide research trends on medicinal plants. International journal of environmental research and public health. 2020; 17: 3376.
- Makinde SCO, Ojekale AB, Oshinaike TS, Awusinu TS. An ethnomedical and ethnobotanical survey of plants herbal therapy used for obesity, asthma, diabetes and fertility by the Badagry people of Lagos state, Nigeria. Journal of Medicinal Plants Studies. 2015; 3: 01-06.
- Hussain F, Pathan S, Sahu K, Gupta BK. Herbs as cosmetics for natural care: A review. GSC Biological and Pharmaceutical Sciences. 2022; 19: 316-322.
- El-Zahar H, Menze ET, Handoussa H, Osman AK, El-Shazly M, et al. UPLC-PDA-MS/MS profiling and healing activity of polyphenol-rich fraction of Alhagi maurorum against oral ulcer in rats. Plants. 2022; 11: 455.
- Yuan C, Jiang B, Xu X, Wan Y, Wang L, et al. Anti-human ovarian cancer and cytotoxicity effects of nickel nanoparticles greensynthesized by Alhagi maurorum leaf aqueous extract. Journal of Experimental Nanoscience. 2022; 17: 113-125.
- Ahmad N, Bibi Y, Raza I, Zahara K, Khalid N, et al. Traditional uses and pharmacological properties of Alhagi maurorum: A review. Asian Pacific Journal of Tropical Disease. 2015; 5: 856-861.
- Bibi Y, Nisa S, Chaudhary FM, Zia M. Antibacterial activity of some selected medicinal plants of Pakistan. BMC complementary and alternative medicine. 2011; 11: 1-7.
- Prabhavathi RM, Prasad MP, Jayaramu M. Studies on qualitative and quantitative phytochemical analysis of Cissus quadrangularis. Advances in Applied Science Research. 2016; 7: 11-17.
- Sarah QS, Anny FC, Misbahuddin M. Brine shrimp lethality assay. Bangladesh Journal of Pharmacology. 2017; 12: 186-189.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis. 2016; 6: 71-79.
- Fatima A, Gupta VK, Luqman S, Negi AS, Kumar JK, et al. Antifungal activity of Glycyrrhiza glabra extracts and its active constituent glabridin. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2009; 23: 1190-1193.
- Siddiqui R, Alam MM, Amin MR, Daula ASU, Hossain MM. Screening of antimicrobial potential and brine shrimp lethality bioassay of the whole plant extract of Spilanthes paniculata Wall. ex DC. Stamford Journal of Microbiology. 2013; 3: 1-5.
- Ahmad N, Shinwari ZK, Hussain J, Perveen R. Phytochemicals, antibacterial and antioxidative investigations of Alhagi maurorum medik. Pakistan Journal of Botany. 2015; 47: 121-124.
- Panthi M, Subba RK, Raut B, Khanal DP, Koirala N. Bioactivity evaluations of leaf extract fractions from young barley grass and correlation with their phytochemical profiles. BMC complementary medicine and therapies. 2020; 20: 1-9.
- Jaberian H, Piri K, Nazari J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food chemistry. 2013; 136: 237-244.
- Shinwari ZK, Ahmad N, Hussain J, Rehman NU. Antimicrobial evaluation and proximate profile of Nepeta leavigata, Nepeta kurramensis and Rhynchosia reniformis. Pakistan Journal of Botany. 2013; 45: 253-259.
- Kadhim MJ, Mohammed GJ, Hameed IH. In vitro antibacterial, antifungal and phytochemical analysis of methanolic extract of fruit Cassia fistula. Oriental Journal of Chemistry. 2016; 32: 1329-1346.