Research article
Volume 2, Issue 4
The Effect of Polysaccharide Extracted from Raw Atractylodes Macrocephala on Loperamide-Induced Constipation in Rats
Wenli Zhu1; Xiaoqing Chi2*
1Hangzhou Ninth People’s Hospital, Hangzhou, Zhejiang 311200, China.
2College of Pharmaceutical Sciences, Zhejiang University, China
Corresponding Author :
Xiaoqing Chi
Tel: +86-17816890619
Email: tiffany1127@zju.edu.cn
Received : Mar 06, 2023 Accepted : Apr 12, 2023 Published : Apr 19, 2023 Archived : www.meddiscoveries.org
Citation: Zhu W, Chi X. The effect of polysaccharide extracted from raw atractylodes macrocephala on loperamide-induced constipation in rats. Med Discoveries. 2023; 2(4): 1033.
Copyright: © 2023 Chi X. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective: To explore the effect and mechanism of Polysaccharide extracted from raw Atractylodes Macrocephala on Loperamide-induced constipation in rats.
Methods: The rats were given Loperamide (3 mg/kg) by intragastric to induce constipation, after that, the rats were administrated the water, ethanol extract and polysaccharides extracted from raw Atractylodes Macrocephala. The feces moisture content, the fecal grains in 12 hours, and the intestinal propulsion rate were measured to evaluate the effect on treating constipation. The ELISA was to assess the levels of motilin (motilin, MTL) and vasoactive intestinal peptide (VIP) in rat plasma, and hematoxylin-eosin (HE) staining was to assess histopathological changes of the colon, the immunohistochemical assay was to assess the expression of mucoprotein 2 (MUC2) and zonula occludens-1 (ZO-1) proteins in the colon tissue.
Results: The results showed that the water extracted from raw Atractylodes Macrocephala could significantly improve the decrease caused by constipation of fecal moisture content, fecal grains in 12 hours and the intestinal propulsion rate in rats. The ethanol extracted from raw Atractylodes Macrocephala had no significant improvement effect. The polysaccharides extracted from Atractylodes Macrocephala significantly increased the level of MTL and decreased the level of VIP in the plasma of constipated rats (P<0.001), improved the colonic mucosal damage and the reduction of goblet cells in constipated rats, and increased the protein expression of MUC2 and ZO-1 in colon tissue of constipated rats (P<0.05). The water extracted of Atractylodes Macrocephala could effectively treat constipation. As Polysaccharides are the active components in treating constipation, it may be through increasing the expression levels of MUC2 and ZO-1 proteins in colon tissue, regulating the levels of MTL and VIP in plasma.
Conclusion: In conclusion, the Polysaccharide could promote gastrointestinal peristalsis, enhance the barrier function of the colonic mucosa, improves constipation.
Keywords: Atractylodes Macrocephala Koidz; Constipation; Polysaccharide; Mucoprotein 2; Zonula Occludens-1.
Introduction
Constipation is a common gastrointestinal disorder, mainly manifested as difficulty in defecation, decreased frequency of defecation, or dry stool [1]. Long-term and repeated constipation seriously affects the work and life of patients. At present, western medicine mostly uses laxatives and prokinetic drugs to treat constipation. Abdominal pain, laxative colon, melanosis of the colon, and severe drug dependence can damage the patient’s enteric nervous system, thereby aggravating constipation. Traditional Chinese medicine has a long history of treating constipation [2]. Traditional Chinese medicine suggests that the people with deficiency of vital energy are unable to digest, and the people with deficiency of spleen vital energy will cause weak intestinal conduction, and dross will stop inside and cause constipation. Atractylodes Macrocephala is the rhizome of Atractylodes Macrocephala Koidz. It is bitter, warm in nature, and returns to the spleen and stomach meridians [3]. Atractylodes rhizome only or in large doses has a significant effect on treating constipation, but the laxative effect and effective substances of Atractylodes Macrocephala are still unclear.
Loperamide is a known drug used clinically to treat acute and chronic diarrhea. It can significantly reduce gastrointestinal transit and defecation frequency. After intragastric administration of Loperamide to rats, it can reduce the feces moisture content and the fecal grains in 12 hours, and reduce intestinal propulsion rate [4]. The rate is relatively similar to the constipation symptoms of clinical patients, and it is a commonly used drug for establishing animal models of constipation [5]. Therefore, the study was used Loperamide to rats to induce constipation, and different polar extracts and components of Atractylodes Macrocephala were administered into the stomach, and the active components of Atractylodes Macrocephala for treating constipation were screened through the indicators of rat defecation, intestinal motility, and colonic histopathology, to provide a scientific basis for the clinical application of Atractylodes Macrocephala.
Materials and methods
Animals
SPF SD male rats, 180~220 g, were purchased from Vitalriver Co., Ltd., animal license number SCXK 2021-0006. The rats were kept in the Animal Experiment Center of Hangzhou Ninth People’s Hospital. Room temperature 20~25°C, relative humidity (50 ± 10) %, and light time 8: 00 ~ 18: 00, free access to food and water, and adaptive feeding for 7 days. Animal experiments were approved by the Experimental Animal Ethics Committee of the Institute of Hangzhou Ninth People’s Hospital.
Chemicals
Raw Atractylodes Macrocephala (Batch No. 20211226) were purchased from Hangzhou Huadong Chinese Herbal Pieces Co., Ltd. Loperamide (Batch No. PHR1162) was purchased from Merck, USA; Mosapride (Batch No. 112885413) was purchased from Alfa Chemistry Co., Ltd.; Gum Arabic (Batch No. 729J022) and Bovine Serum Albumin (BSA) (Batch No. 1106F052) were purchased from Beijing SoLarbio Technology Co., Ltd. (Beijing, China); Activated Carbon (Batch No. C12535790) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China); 4 % Paraformaldehyde Universal Tissue Fixative (Batch No. 20211201) was purchased from Beijing Lanjie Ke Technology Co., Ltd. (Beijing, China); Rat Motilin (MTL) (Batch No. H182-1-2), Vasoactive Intestinal Peptide (VIP) ELISA kit (Batch No. H219) were purchased from Nanjing Jiancheng Institute of Bioengineering and Technology (Nanjing, China); Mucin 2 (Mucoprotein2, MUC2) antibody (Batch No. GR3374627-13), Zonula Occludens-1 (Zonula Occludens-1, ZO-1) antibody (Batch No. GR3427980-4), HRP-Labeled Goat Anti-Rabbit (Batch No. GR3265468-5) were purchased from Abcam, UK; Xylene (Batch No. 20220320), Phenol (Batch No. 20220422), Concentrated Sulfuric Acid (Batch No. 20210710), Absolute Ethanol (Batch No. 20210710), 95 % Ethanol (Batch No. 20211110), Acetone (Batch No. 20210320) were purchased from Sinopharm Chemical Reagent Co., Ltd.; Chloral Hydrate (Batch No. H1821088) and Glucose Standard (Batch No. L2009312) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
Instruments
Microplate Reader (Varioskan Flash, Thermo); U-2910 Ultraviolet Spectrophotometer (U-2910, Hitachi); High-Speed Low-Temperature Centrifuge (Micro17R, Thermo); Electric Blast Drying Box (DHG-9070A, Shanghai Heng Science); Crushing Machine (YF-111B, Ruian Yongli Pharmaceutical Machinery); Thermostat Electric Heating Mantle (DZTW, Beijing Yongguangming Medical); Circulating Water Vacuum Pump (SHK-III, Zhengzhou Ketai Experimental); Low-Temperature Rotary Evaporator (R200, BUCHI); Freeze Dryer (LGJ-185, Beijing Songyuan Huaxing Technology); Pathological Slicer (RM2235, Leica); Embedding Machine (JBP7, Wuhan Junjie); Roasting Machine (DB-B2, Changzhou Guohua); Imaging System CCD Camera (MIchrome 5 Pro, Fuzhou Xintu Optoelectronics).
Methods
Polysaccharides preparation
Water extracted from Raw Atractylodes Macrocephala Preparation: 500 g raw Atractylodes Macrocephala was dissolve in 5 L distilled water. Soaked for 12 hours, and heated for 2 hours, then filtered while the water hot (15 layers of gauze), repeated twice, and combined the filtrates [6]. Vacuum filtered and concentrated after low-pressure, following placed in a cold Freezedrying machine to obtain 365.2 g of freeze-dried, the yield was 73.04 %.
Ethanol extracted from Raw Atractylodes Macrocephala Preparation: 500 g raw Atractylodes Macrocephala was dissolve in 5 L 95% ethanol. Soaked for 12 hours, and heated for 2 hours, then filtered while the solution hot (15 layers of gauze), repeated twice, and combined the filtrates [7]. Vacuum filtered and concentrated after low-pressure, following placed in a cold Freeze-drying machine to obtain 63.9 g of freeze-dried, the yield was 12.78%.
Polysaccharides extracted from Raw Atractylodes Macrocephala Preparation: 500 g raw Atractylodes Macrocephala was dissolve in 5 L distilled water. Soaked for 12 hours, and heated for 2 hours, then filtered while the solution hot (15 layers of gauze), repeated twice. Combined the filtrates after low-pressure to obtain 0.2 g/mL solution. 95% ethanol was used to ethanol precipitation, and the final volume fraction of ethanol precipitation was 70%. Vacuum filtered after placed at 4°C for 24 h, repeated twice and combined the filtrates [8]. The precipitate obtained by ethanol precipitation was washed with acetone and absolute ethanol three times, and then freeze-dried to obtain Atractylodes Polysaccharide with a yield of 52.3 %.
Polysaccharides concentration measurement
Standard Curve Preparation: 10.07 mg glucose was dissolved in the distilled water to prepare 50.00 mL stock solution. 0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0 mL stock solution was added to 10 mL volumetric flasks with distilled water, respectively. 1.0 mL was added to the stoppered test tube with 1.0 mL 5% phenol and 5.0 mL concentrated sulfuric acid, placed at room temperature [9]. Measured the absorbance (A) at 490 nm. Linear regression: the concentration was as the abscissa (X) and A was as the ordinate (Y).
Polysaccharides concentration measurement: Appropriate water and polysaccharide extracted from raw Atractylodes Macrocephala were dissolved in distilled water in 50 mL measuring bottles. 1.0 mL was added to the stoppered test tube with 1.0 mL 5% phenol and 5.0 mL concentrated sulfuric acid, placed at room temperature [10]. Measured the absorbance (A) at 490 nm, and calculated the polysaccharide concentration according to the standard curve.
Models
Rats were administrated Loperamide (3 mL/kg) (i.g.) for 14 consecutive days at 8:00 and 14:00 to induce constipation. The constipation rats were evaluated by feces properties, feces moisture content, and feces grains. The rats in the control group were given equal volume of saline.
Grouping and administration
Experimental 1: 40 rats were divided into 5 groups, 8 rats in each group: control group, constipation group, Mosapride group (3 mg/kg), Atractylodes Macrocephala water group (6 mg/kg), Atractylodes Macrocephala ethanol extract group (6 mg/kg). After administrated Loperamide for 14 days, the rats in group 3, 4, 5 were given (i.g.) Mosapride (3 mg/kg), Atractylodes Macrocephala water (6 mg/kg), Atractylodes Macrocephala ethanol extract (6 mg/kg), and the rats in group 1 and 2 were given (i.g.) the same volume of saline, once a day for 14 consecutive days.
Experimental 2: 32 rats were divided into 4 groups, 8 rats in each group: control group, constipation group, Mosapride group (3 mg/kg), Atractylodes Macrocephala Polysaccharides group (6 mg/kg). After administrated Loperamide for 14 days, the rats in group 3, 4were given (i.g.) Mosapride (3 mg/kg), Atractylodes Macrocephala Polysaccharides (6 mg/kg), and the rats in group 1 and 2 were given (i.g.) the same volume of saline, once a day for 14 consecutive days.
Sampling and preparation
All rats were fasted without food and water for 12 hours after the last administration, anesthetized by 4 % chloral hydrate (i.p.), blood was collected from the abdominal aorta, and placed in blood collection tubes containing heparin sodium anticoagulant, 3000 r/min centrifuged at 4OC for 20min. The supernatant was collected and stored at -80OC. Collected 2 cm of colonic tissue and cleaned with normal saline, dried with paper and fixed in 4 % paraformaldehyde universal tissue fixative for hematoxylin-eosin (HE) staining and Immunohistochemical detection.
Feces moisture content and feces grains measurement
Fresh feces from rats in each group were collected on Day 0, 7, 14, 21, and 28, dried at 90°C for 3 h. Weighed before and after drying to obtain the wet and dry weight of the feces, and calculated the moisture content of the feces.
Feces Moisture Content = (Wet weight - Dry weight)/Wet weight
Collected the feces of rats in each group for 12 hours on Day 0, 7, 14, 21, and 28, and counted the feces grains.
Intestinal propulsion
After the last administration, the rats in each group were fasted with water for 12 hours, 2 mL of 10% activated carbon solution (10% gum arabic powder was boiled and fully dissolved until transparent, then added 10% activated carbon powder and stirred well) was administrated (i.g.), after 20 min, the rats was following anesthetized. The intestine from the pylorus to the cecum was collected to measure the propulsion rate.
Intestine Propulsion Rate = Activated Carbon Propulsion Distance/Total Length of Intestine
MTL and VIP in the plasma measurement
The levels of MTL and VIP in the plasma of rats in each group were measured according to the kits instructions [11,12].
Colon pathology
The colon was dissected and fixed in 4% paraformaldehyde solution for HE staining, and the pathological changes of the colon were observed under the microscope.
MUC2 and ZO-1 Expression
1 cm of colon tissues from rats in each group was washed with normal saline and fixed in 4% paraformaldehyde solution. Dehydrated with gradient ethanol, changed transparent, soaked with wax, embeded with paraffin, sliced, and dried, then dewaxed with xylene, hydrated with gradient ethanol. After that, 3% BSA was added to incubate at room temperature for 30 min, then added MUC2 antibody (1:500) and ZO-1 antibody (1:100), and incubated overnight at 4°C. On the other day, washed slides with PBS 3 times (each time for 5 min) and added HRP-Labeled Goat Anti-Rabbit (1:500), and incubated at 37°C for 30 min. Washed the slides 3 times with PBS (each time for 5 min), and added DAB to incubate protected from light for 3 min. Washed with distilled water for 5 min, and stained with hematoxylin for 5 min [13,14]. After dehydration and transparent, neutral gum was used to mounting. Image-Pro Plus 6.0 image analysis system to analyze the expression of MUC2 and ZO-1 on immunohistochemical images.
Statistics and analysis
One-way ANOVA of SPSS Statistics for Mac (Version 20) was used for statistical analysis of the data, followed by Tukey’s post hoc test for multiple comparisons between groups. The results were presented as the means ± S.D. All statistical analyses were performed using the GraphPad InStat software (Version 7, GraphPad). P value < 0.05 or P value < 0.01 was considered statistically significant.
Results
Polysaccharides concentration measurement
Phenol-sulfuric acid method was used to detect the polysaccharide content of Atractylodes Macrocephala polysaccharides. The standard curve was Y=9.484 X+0.1029, R2=0.9991. The calculated polysaccharide mass fraction in the water extract of Atractylodes Macrocephala was 69.00%, and the polysaccharide mass fraction of Atractylodes Macrocephala polysaccharides was 89.30%.
The effect comparison of water and ethanol extract of atractylodes macrocephala on constipation
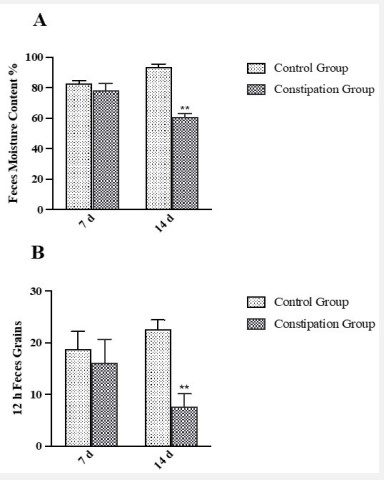
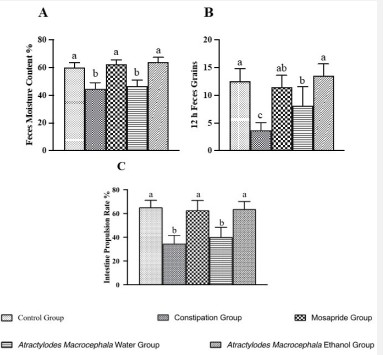
Feces grains and moisture content are important indicators to evaluate the gastrointestinal function of constipation animals, while feces grains represent the strength of gastrointestinal motility, and the moisture content represents the absorption and secretion of intestinal water [15]. In this study, the rat was administrated Loperamide (i.g.) to induce constipation. The results showed that the feces moisture content and feces grains in 12 hours of rats in the constipation group were decreased after administrated Loperamide for 7 days, but there was no significant difference compared with the control group (Figure 1A, 1B). The feces moisture content and feces grains in 12 hours of rats in the constipation group were decreased after administrated Loperamide for 14 days (P<0.001), indicated that the constipation model was successfully replicated. After the constipation rats were treated with water and ethanol extract that extracted from Atractylodes Macrocephala for 14 days, compared with the constipation group, the water extract of Atractylodes Macrocephala group significantly increased the feces moisture content, feces grains in 12 hours and small intestine propulsion rate of rats (P<0.01), and there was no significant difference compared with the Mosapride group. However, the ethanol extract has no obvious effect on the defecation and small intestine propulsion rate of constipation rats (Figures 2A, 2B, 2C).
A: The results of the feces moisture content of rats after administrated Loperamide for 7 and 14 days;
B: The results of feces grains in 12 hours of rats after administrated Loperamide for 7 and 14 days. Bars with * or ** are significantly different (P < 0:05 or P < 0:01).
A: The results of the feces moisture content of rats after administrated;
B: The results of feces grains in 12 hours of rats after administrated; C: The results of intestine propulsion rate of rats after administrated. Bars with different letters are significantly different (P < 0:05 or P < 0:01).
The effect of atractylodes macrocephala polysaccharides on constipation
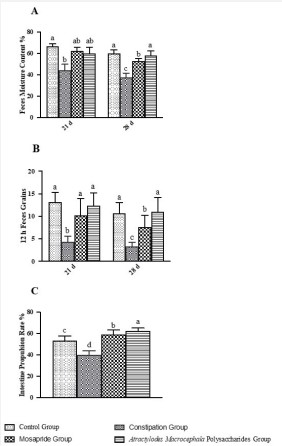
The results showed that after the constipation rats were treated with Atractylodes Macrocephala polysaccharide for 7 days, the feces moisture content and feces grains in 12 hours had shown an upward trend and was close to the control group. After the constipation rats were treated with Atractylodes Macrocephala polysaccharide for 14 days, the feces moisture content, feces grains in 12 hours and small intestine propulsion rate of rats were significantly increased (P<0.001), closed to the level of normal rats, and the effect was equivalent compared with the positive drug Mosapride group (Figure 3). It suggested that the of Atractylodes Macrocephala polysaccharide could improve the situation of constipation rats significantly that the polysaccharide might be the effective component of raw Atractylodes Macrocephala in treating constipation.
The effect of atractylodes macrocephala polysaccharides on MLT and VIP in plasma on constipation
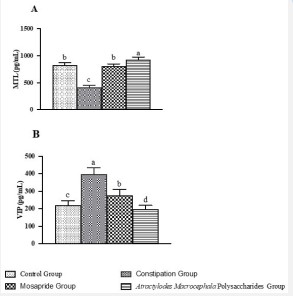
MTL is an excitatory neurotransmitter that regulates gastrointestinal function, and can cause strong contraction of stomach and obvious peristalsis of small intestine [16]. VIP is one of the gastrointestinal inhibitory neurotransmitters, which can relax gastrointestinal smooth muscle and inhibit gastrointestinal peristalsis [17]. As shown in Figure 4, compared with the control group, the plasma MTL level of rats in the constipation group was significantly decreased (P<0.05) and the VIP level was significantly increased (P<0.01). However, Atractylodes Macrocephala Polysaccharide could significantly increase the plasma MTL level (P<0.001) and decreased the plasma VIP level (P<0.001) of constipation rats, the treatment effect of polysaccharide group was significantly treated than that of positive drug Mosapride group (P<0.05).
A: The results of the feces moisture content of rats after administrated;
B: The results of feces grains in 12 hours of rats after administrated;
C: The results of intestine propulsion rate of rats after administrated. Bars with different letters are significantly different (P < 0:05 or P < 0:01).
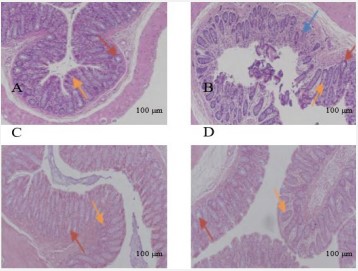
The effect of atractylodes macrocephala polysaccharides on pathological of colon on constipation
As shown in Figure 5, the colon mucosa of rats in the control group was intact, the glands were placed intactly, the structure was clear, and no abnormal pathological changes were found. In the constipation group, the mucosal epithelium of colon was severely damaged, the crypts were disordered, the number of goblet cells of intestinal gland was significantly reduced, and inflammatory cell infiltration was accompanied. After the treatment of Atractylodes Macrocephala Polysaccharide group and positive drug Mosapride group, the structure of epithelial cells in the colonic mucosa of rats was intact, the arrangement of glands tended to be normal, and the goblet cells were obvious increased, which was significantly improved compared with the constipation group.
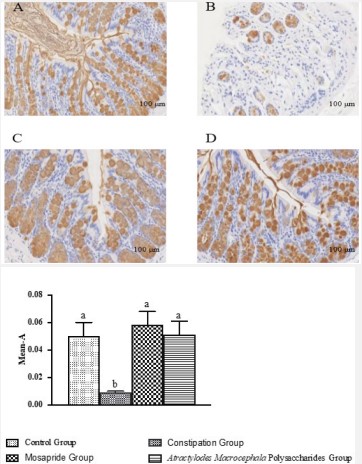
The effect of atractylodes macrocephala polysaccharides on MUC2 expression on constipation
MUC2 is one of the most important glycoproteins that form the chemical barrier (mucus layer) of intestinal mucosa. It forms a mucus layer on the surface of the intestine to play the role of lubricating the intestine [18]. As shown in Figure 6, MUC2 was positive in colon tissue of rats in each group, and the cytoplasmic staining granules were light brown, brown yellow or brown. Compared with the control group, the expression of MUC2 in the colon tissue of the constipation group was significantly decreased (P<0.001); After treatment with Atractylodes Macrocephala Polysaccharide and Mosapride, the expression of MUC2 in colon tissue of rats was significantly increased than constipation group (P<0.001). It was closed to the level of normal rats, and the efficacy of the two drugs was equivalent.
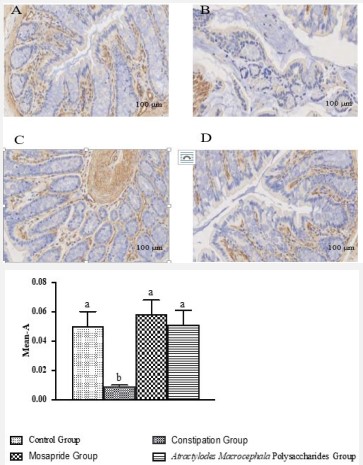
The effect of atractylodes macrocephala polysaccharides on ZO-1 expression on constipation
ZO-1 is an important structural protein that constitutes the tight junction of intestinal epithelial cells, and has an important regulatory role in the process of material trans-epithelial transport, cell proliferation and differentiation [19]. The low expression in intestinal tissue will lead to the reduction of the function of intestinal mucosal cell tight junction protein, affect the integrity of intestinal mucosal epithelial barrier, cause the intestinal hypotonic environment, intestinal water loss, and cause constipation. As shown in Figure 7, ZO-1 was positive in colon tissue of rats in each group, and was light brown or brown. Compared with the control group, the expression of ZO-1 in colon tissue of constipation group was significantly decreased (P<0.001); After the treatment of Atractylodes Macrocephala Polysaccharide and Mosapride, the expression of ZO-1 increased significantly (P<0.01).
Discussion
Atractylodes Macrocephala is specially used in the two meridians of the spleen and stomach as the “first medicine for invigorating the spleen”[20]. It can not only strengthen the spleen function, but also relieve the spleen damage. Since ancient times, it has been used by doctors of all dynasties to regulate diseases in the function of the spleen and stomach. The raw Atractylodes Macrocephala can be used alone or in combination with the prescription, which can help to improve the blood circulation and clear the blood, reduce the turbidity, regulate the mechanism smoothly, distribute the body fluid, promote the blood circulation, and smooth the gastrointestinal peristalsis, so that constipation can be eliminated automatically [21,22]. However, the effective mechanism of Atractylodes Macrocephala in treating constipation is still unclear.
The decrease of intestinal peristalsis caused by insufficient gastrointestinal motility and disturbance of intestinal water and lubricating mucus secretion is the main cause of constipation[23]. In this study, Loperamide hydrochloride was selected for modeling by inhibiting the contraction of intestinal smooth muscle, reducing intestinal peristalsis, prolonging the retention time of intestinal contents, and reducing the difficulty of defecation caused by the secretion of digestive fluid. The feces grains, feces moisture content and small intestine propulsion rate are important indicators to evaluate gastrointestinal function[24]. Hu et al. found that the feces of constipation rats were dry and hard, and the feces grains and small intestine propulsion rate were significantly reduced [25]. In this study, the effects of water extract and ethanol extract of Atractylodes Macrocephala on constipation rats were compared by observing the feces moisture content, the feces grains in 12 hours and the small intestine propulsion rate. The results showed that the water extract of Atractylodes Macrocephala could significantly improve the symptoms of constipation rats, while the ethanol extract had no obvious effect. Polysaccharide has the effects of protecting gastrointestinal mucosa, balancing intestinal flora, improving intestinal environment, etc., and plays a very important role in moistening intestines and relieving constipation. The water extract of Atractylodes Macrocephala mainly contains polysaccharides (fraction: 69.00%). Therefore, the polysaccharide of Atractylodes Macrocephala (fraction of 89.30%) was prepared from the water extract of Atractylodes Macrocephala by traditional methods. Its therapeutic effect on constipation was evaluated on the model of constipation induced by Loperamide in rats. It was found that the polysaccharide could significantly improve the symptoms of constipation rats and repair the pathological damage of colon tissue caused by constipation. In order to further verify its effect, the therapeutic effect of polysaccharides on constipation rats was investigated from the levels of MTL and VIP in plasma and the expression of MUC2 and ZO-1 protein in colon tissue.
Brain gut peptide is a kind of small molecular polypeptide substances secreted by endocrine cells of central nervous system, gastric nervous system and gastrointestinal tract. Brain gut peptide is the material basis and important target of brain-gut axis, and plays a key role in the regulation of gastrointestinal function [26,27]. Under physiological conditions, brain gut peptide regulates gastrointestinal motility through the central nervous system and gastrointestinal smooth muscle cells [28]. According to the mechanism of action, brain gut peptide can be divided into excitatory neurotransmitters and inhibitory neurotransmitters [29]. MTL is an excitatory neurotransmitter that regulates gastrointestinal function and can cause strong contraction of the stomach and obvious peristalsis of the small intestine, while VIP is one of the gastrointestinal inhibitory neurotransmitters, mainly distributed in the whole layer of the intestine, and has the function of relaxing gastrointestinal smooth muscle and inhibiting gastrointestinal peristalsis [30]. Studies have confirmed that the VIP level in the serum of constipation rats was significantly increased, and the wall-breaking decoction of Atractylodes Macrocephala could regulate gastrointestinal movement by reducing the VIP level of constipation mice [31]. Fang, et al have found that the serum MTL level of Loperamide induced constipation rats was significantly reduced, and the VIP level was significantly increased [32]. The results of this study were consistent with the above reports. At the same time, it was also found that Atractylodes Macrocephala Polysaccharide could significantly regulate the plasma MTL and VIP levels in constipation rats, so as to promote gastrointestinal peristalsis and thus played a role in the treatment of constipation.
MUC2 is a kind of high relative molecular weight glycoprotein secreted by epithelial cells, and also the most important glycoprotein involved in the formation of intestinal mucosal barrier. This protein forms a mucous layer on the intestinal surface to play the role of lubricating and antagonizing the intestinal adhesion and invasion of pathogenic bacteria [33]. ZO-1 is widely distributed in tissues and organs such as intestines and vascular endothelium. It is an important structural protein that constitutes the tight junction of epithelial cells, and plays an important role in regulating the process of material transepithelial transport, cell proliferation and differentiation. In addition, ZO-1 is the hub of tight junction between cells [34]. Once ZO-1 is broken, the structure and function of tight junction will change accordingly. Therefore, ZO-1 is often used to observe the index of intestinal tight junction barrier function and permeability function, and ZO-1 is likely to be one of the targets of tonifying spleen traditional Chinese medicine. Mei Lu et al. found that the expression of MUC2 and ZO-1 mRNA in the colon of mice with constipation induced by Loperamide was significantly reduced. The results of this study were consistent with the results of the above reports. At the same time, this study was also found that polysaccharides could significantly increase the expression level of MUC2 and ZO-1 protein in the colon of rats with constipation induced by Loperamide, enhance the function of colonic mucosal barrier, and play a role in alleviating constipation.
This study was found that the water extract of Atractylodes Macrocephala has a clear therapeutic effect on constipation, while polysaccharide could repair the pathological damage of colon tissue caused by constipation, regulate the plasma MTL and VIP levels of constipation rats, and improve the low expression of MUC2 and ZO-1 protein in colon tissue of constipation rats. It was suggested that polysaccharide may be the main effective component of raw Atractylodis Macrocephala in the treatment of constipation. However, it was still unclear whether there was a small molecular effective component in the water extract of Atractylodes Macrocephala for constipation treatment except polysaccharide. The differences in the efficacy of the water extract of Atractylodes Macrocephala and polysaccharide components will be compared in the future to provide a reference for determining the effective components of Atractylodes Macrocephala for constipation treatment.
Interest conflicts: All authors declared that there was no conflict of interest.
References
- Elsagh M, Behbahani FA, Fartookzadeh MR, Adibi P. Categorization of Functional Constipation in Traditional Persian Medicine. Journal of Evidence-Based Complementary & Alternative Medicine. 2015; 23: 105-115.
- Lindley LC, Keim-Malpass J, Cozad MJ, et al. A National Study to Compare Effective Management of Constipation in Children Receiving Concurrent Versus Standard Hospice Care. Journal of Hospice & Palliative Nursing. 2022; 24: 70-77.
- Lin Z, Xiao Z, Zhu D, Yan Y,Yu B, Wang Q, et al. Aqueous extracts of FBD, a Chinese herb formula composed of Poria cocos, Atractylodes macrocephala, and Angelica sinensis reverse scopolamine induced memory deficit in ICR mice. Pharmaceutical Biology. 2009; 47: 396-401.
- Raquel IA. Radiographic dose-dependency study of loperamide effects on gastrointestinal motor function in the rat. Temporal relationship with nausea‐like behavior. Neurogastroenterology and motility. 2019; 31: 555-563.
- Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T, et al. Sulfated Polysaccharides, but Not Cellulose, Increase Colonic Mucus in Rats with Loperamide-Induced Constipation. Digestive Diseases & Sciences. 2001; 46: 1482-1489.
- Shan GS, Zhang LX, Zhao QM, Xiao HB, Zhuo RJ, et al. Metabolomic study of raw and processed Atractylodes macrocephala Koidz by LC-MS. Journal of Pharmaceutical and Biomedical Analysis. 2014; 98: 74-84.
- Ha H, An H, Shim KS, Kim T, Lee KJ, et al. Ethanol Extract of Atractylodes macrocephala Protects Bone Loss by Inhibiting Osteoclast Differentiation. Molecules. 2013; 4: 1-6.
- Ming W U, Han D, Guo LQ. Ultrasonic-assisted Extraction and Antioxidant Activities of Polysaccharides from Atractylodes macrocephala. Food Research and Development. 2015; 22: 175-182.
- Li LL, Yin FG, Zhang B, Peng HZ, Li FN, Zhu NS, et al. Dietary supplementation with Atractylodes Macrophala Koidz polysaccharides ameliorate metabolic status and improve immune function in early-weaned pigs. Livestock Science. 2011; 142: 33-41.
- Ji J X. Macrophage activation by polysaccharides from Atractylodes macrocephala Koidz through the nuclear factor-kappa B pathway. Pharmaceutical Biology. 2015; 53: 53-56.
- Suo H, Zhao X, Qian Y. Therapeutic Effect of Activated Carbon-Induced Constipation Mice with Lactobacillus fermentum Suo on Treatment. International Journal of Molecular Sciences. 2014; 15: 21875-21895.
- Huang J, Li S, Wang Q. Pediococcus pentosaceus B49 from human colostrum ameliorates constipation in mice. Food & Function. 2020; 11: 425-432.
- Reich M, Iwanczak B. Constipation in children - Causes, diagnostics and treatment. Advances in Clinical & Experimental Medicine. 2007; 16: 443-456.
- Hong MY, Viviana Y, Shirin H. Effects of Mango Consumption on Total Antioxidant Capacity, Gut Permeability Proteins (ZO-1, Claudin-2, and Occludin), and Bowel Movement Habits. Current Developments in Nutrition. 2022; 737-745.
- Stewart ML, Schroeder NM. Dietary treatments for childhood constipation: efficacy of dietary fiber and whole grains. Nutrition Reviews. 2013; 1: 98-109.
- Liu SY, Liu Y, Yao JY. Effects of Traditional Chinese Medicine Fomentation on Patients of Functional Constipation. Progress in Modern Biomedicine, 2014; 5: 921-928.
- He J. Study on the relationship between the slow transit constipation and VIP, SP nerves in rat colon myenteric nerve plexus. Digestive Surgery. 2015; 343: 1-8.
- Garg P, Ravi A, Patel NR. Matrix metalloproteinase-9 regulates MUC-2 expression through its effect on goblet cell differentiation. Gastroenterology. 2007; 132: 1877-1889.
- Elias B C, Suzuki T, Seth A. Phosphorylation of Tyr-398 and Tyr402 in Occludin Prevents Its Interaction with ZO-1 and Destabilizes Its Assembly at the Tight Junctions. Journal of Biological Chemistry. 2009; 284: 1559-1569.
- Li B X, Li WY, Tian YB, et al. Polysaccharide of Atractylodes macrocephala Koidz Enhances Cytokine Secretion by Stimulating the TLR4–MyD88–NF- κ B Signaling Pathway in the Mouse Spleen. Journal of Medicinal Food. 2019; 22: 65196-65207.
- Lee JC, Lee KY, Son YO, Choi KC, Kim J, et al. Stimulating effects on mouse splenocytes of glycoproteins from the herbal medicine Atractylodes macrocephala Koidz. Phytomedicine. 2007; 14: 390-395.
- Cai B, Cai H, Xu Z. Study on chemical fingerprinting of crude and processed Atractylodes macrocephala from different locations in Zhejiang province by reversed-phase high-performance liquid chromatography coupled with hierarchical cluster analysis. Pharmacognosy Magazine. 2012; 8: 300-307.
- Qiao L, Wang LJ, Wang Y. A Randomized, Double-Blind, and Placebo-Controlled Trial of Chinese Herbal Medicine in the Treatment of Childhood Constipation. Clinical and Translational Gastroenterology. 2021; 12: e00345.
- Ying C, Xu J, Ren Q. The Effect of Millet Porridge on the Gastrointestinal Function in Mice. Journal of Food and Nutrition Research. 2018; 6: 152-157.
- Can-Ji Hu, Xiao TC, Zhang PF. Effect of Compound Fruit and Vegetable Tablet on Relieving Constipation and Defecating Feces Excretion in Mice[J]. DEStech Transactions on Biology and Health. 2018; 51: 279-286.
- Dockray GJ, Vaillant C, Williams RG . New vertebrate brain|[ndash]|gut peptide related to a molluscan neuropeptide and an opioid peptide. Nature. 1981; 293: 656-657.
- Liu Q, Li F, Huang L. FumDSB Can Reduce the Toxic Effects of Fumonisin B1 by Regulating Several Brain-Gut Peptides in Both the Hypothalamus and Jejunum of Growing Pigs. Toxins. 2021; 13: 874-902.
- Giraud AS, Dockray GJ, Williams RG. Immunoreactive Met-Enkephalin Arg6 in Rat Brain, and Bovine Brain, Gut, and Adrenal. Journal of Neurochemistry. 2010; 43: 1236-1242.
- Miller, Gary D. Appetite Regulation: Hormones, Peptides, and Neurotransmitters and Their Role in Obesity. American Journal of Lifestyle Medicine. 2017; 155982761771637.
- Wang R, Peng S, Zhou Y. Preventive effect of Dendrobium candidum Wall. ex Lindl. on activated carbon-induced constipation in mice[J]. Experimental and therapeutic medicine. 2015; 9: 563-568.
- Mi S, Soo-Kyoung L, Wang JH. The Root of Atractylodes macrocephala Koidzumi Prevents Obesity and Glucose Intolerance and Increases Energy Metabolism in Mice. International Journal of Molecular Sciences. 2018; 19: 1220-1226.
- Wang L, Liu J, Zhang Y. Effects of Laparoscopic TME Combined with Transanal ISR in Treatment of Ultra-low Rectal Cancer and Observation of Postoperative Complications. Medical & Pharmaceutical Journal of Chinese People’s Liberation Army. 2018; 125: 1327-1336.
- Cappello F, Rappa F, David S. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Research. 2003; 23: 1325-1331.
- Zhang B, Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. British Journal of Nutrition. 2009; 102: 687-693.