Research article
Volume 2, Issue 4
Treatment of Melanoma Metastasis: Surgical, Chemotherapy, and Innovation
Chelsea Rosen1; Taeya Mayes1; Claire Overholt1; Brandon Lucke-Wold2*
1University of Florida College of Medicine, Gainesville, FL, USA.
2Department of Neurosurgery, University of Florida, Gainesville, FL, USA.
Corresponding Author :
Brandon Lucke-Wold
Email: Brandon.Lucke-Wold@neurosurgery.ufl.edu
Received : Mar 02, 2023 Accepted : Apr 07, 2023 Published : Apr 14, 2023 Archived : www.meddiscoveries.org
Citation: Rosen C, Mayes T, Overholt C, Lucke-Wold B. Treatment of melanoma metastasis: Surgical, chemotherapy, and innovation. Med Discoveries. 2023; 2(4): 1032.
Copyright: © 2023 Lucke-Wold B. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Melanoma is a form of skin cancer with an increased ability to metastasis to organs such as the brain and other visceral organs, contributing to its aggressiveness and seriousness. Melanoma’s prevalence around the globe rapidly continues to rise. Melanoma development is a complex process often depicted as a step-wise process with the potential to end in metastatic disease. Recent studies suggest that the process could be non-linear. Melanoma has many risk factors including genetics, UV exposure, or exposure to carcinogens. Current treatments for metastatic melanoma include surgery, chemotherapy, and immune checkpoint inhibitors (ICIs); however, each of these treatments comes with limitations, toxicities, and relatively poor outcomes. There are various guidelines set by the American Joint Committee on Cancer guiding surgical treatment options based on the site of metastasis. Surgical treatments cannot fully treat widespread metastatic melanoma but can contribute to better patient outcomes overall. Many chemotherapy options are ineffective against melanoma or come with extreme toxicities; however, alkylating agents, platinum analogs, and microtubular toxins have shown some effectiveness against metastatic melanoma. ICIs are a relatively new treatment option and offer a promising option for patients; however, ICIs are subject to tumor resistance mechanisms and are not effective for every metastatic melanoma patient. Due to the limitations of conventional treatments, there is a need for newer and more effective treatment options for metastatic melanoma. This review aims to highlight the current surgical, chemotherapy, and ICI treatments for metastatic melanoma, as well as current clinical and preclinical investigations to discover revolutionary options for patients.
Keywords: Melanoma; Metastasis; Surgery; Chemotherapy; Immune checkpoint inhibitor.
Introduction
Melanoma is a highly aggressive and deadly skin cancer that results from aberrant growth and proliferation of melanocytes, the melanin-producing cells of the epidermis [1,2]. Genetic mutations are the driving force behind this uninhibited proliferation and the subsequent development of melanoma [3]. Common somatic mutations include mutations in NRAS, BRAF, GNAQ, c-KIT, and CDKN2A genes of a melanocyte [3]. Specific mutational frequencies also contribute to the abnormal growth of melanocytes [3].
The ability of melanoma to metastasize attributes to the high rates of morbidity and mortality of melanoma [1]. Metastatic melanoma cells share important antigens with endothelial vasculatures, such as cell adhesion molecules, which contributes to its migration and invasion of distant sites [2]. Although metastatic melanoma cells are highly antigenic, they are well equipped to evade host defenses and stimulate angiogenesis and lymphangiogenesis, leading to successful metastasis [2]. According to the Surveillance, Epidemiology, and End Results (SEER) Program by the NIH, metastatic melanoma tumors significantly impacts patient survival rates: patients diagnosed with localized (stage 1) melanoma had a 5-year relative survival rate of 99.5% while patients diagnosed with distant (stage 4) melanoma had a 31.9% relative survival rate [4].
Treatment options differ based on the stage of melanoma. Primary melanoma is generally treated by wide excision surgical removal, yielding a high survival rate [5]. Later stages of melanoma are related to metastasis and can be difficult to treat. The development of new therapeutic approaches, such as immunotherapy, are designed to target specific mutations, and these approaches are promising for metastatic melanoma treatment [6].
Progression and routes of metastasis
The Clark model of melanoma development details a linear progression based on clinical and histopathological evidence: (1) Common acquired melanocytic nevus; (2) dysplastic melanocytic nevus; (3) radial growth phase; (4) vertical growth phase; (5) metastatic melanoma [7,8]. While this is the widely accepted model of linear, stepwise melanoma progression, it should be noted that newer models suggest metastases can develop in earlier steps and travel to regional or distant sites [8,9].
The first step of melanoma progression is the appearance of the common acquired melanocytic nevus (CMN). CMNs are benign neoplasms due to the proliferation and aggregation of melanocytes [8,9]. The development of these clonal, growtharrested melanocytes can be initiated by oncogenic mutations in the MAPK pathway, most commonly via BRAFV600E-activating mutations [9,10]. CMNs are 2-6 mm, symmetric, uniform moles that can develop at any time and can be found in the epidermis, dermis, or both [5,10,11]. It is important to note that approximately 33% of melanomas are derived from CMNs, but a majority of melanocytic nevi will not progress to melanoma [10].
The second step of melanoma progression involves atypical or dysplastic melanocytic nevi. Common features among dysplastic nevi include uneven borders, large shapes, and multicolored characteristics [7,12]. Dysplastic nevi may also present with enlarged nuclei, dense distribution along the basal layer, and thickening of the epidermal layer [13]. Dysplastic nevi can be graded as mild, moderate, and severe primarily depending on nuclear size, but morphology, euchromatism, and nucleoli prominence also contribute to grading [14,15]. According to Reddy et al., mildly or moderately dysplastic nevi were less likely to become malignant, while severely dysplastic nevi were more likely to progress into malignance and excision could benefit detection and prevention [15].
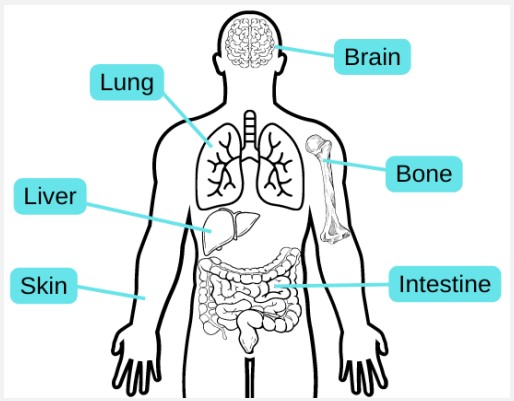
The third and fourth steps of Clark’s model are the Radial Growth Phase (RGP) and the Vertical Growth Phase (VGP), respectively. RGP of primary melanoma involves the formation of a patch or plaque lesion via expansion along the radii of the asymmetric dysplastic nevus [7,16]. During this step, cells may invade the dermis or remain in situ, but a nodule does not form [16]. Lesions identified during this step are linked to a higher risk of melanoma and are therefore removed via surgical excision [7]. Conversely, VGP is associated with invasion into the dermis and tumor formation as it grows down into the dermis and becomes raised [7]. As opposed to the previous steps, a nodule identified during VGP is often symmetrical and homogenous in color [7,16]. Histologically, VGP is often marked by mitoses and a dominant expansile dermal nest composed of neoplastic melanocytes [7,17]. It is important to note that VGP signifies the point at which melanoma becomes capable of metastatic events. Therefore, patients diagnosed at this stage are at heightened risk for metastatic melanoma [17]. The fifth and final step in Clark’s model of melanoma progression is metastatic melanoma. Metastasis is a complex, multistep process that allows melanoma metastases to travel to both regional and distant sites of the body via the blood or lymph vessels [7,9,18]. Regional metastases to the skin can be subclassified as satellite lesions if they are closer to the primary tumor site, or in transit metastases if they are more distant [9]. Distant metastatic sites include the skin, lung, brain, liver, bone, and intestine [2,9]. It is worth noting that although metastatic melanoma is the final step, it will continue to progress and obtain new mutations contributing to malignancy, drug resistance, and increased metastatic disease if left untreated [7].
Epidemiology of melanoma
The worldwide incidence rate of melanoma has increased rapidly over the past few decades [6,9,23]. According to the SEER Program, an estimated 99,780 new cases were diagnosed in the U.S. in 2022, accounting for 5.2% of all new cancer cases [4]. Additionally, approximately 8,000 cancer deaths, or 1.3% of all cancer deaths, in the U.S. resulted from metastatic melanoma [4,24]. Although melanoma metastases can appear in various sites, it seems to display tropism for specific organs including the brain [2,9]. In a study of 26,430 patients with brain metastases, patients with primary melanoma accounted for 28.2% of the brain metastases [25]. In newly diagnosed metastatic melanoma, approximately one-third of patients are likely to also present with brain metastases [25].
The genetic mutations that lead to the development of melanoma can be attributed to a mix of environmental exposure and genetic susceptibility factors [8,23]. The most explicit modifiable link to melanoma is UV radiation exposure from the sun or tanning beds [1,23,24]. UV radiation can cause damage to DNA in melanocytes resulting in dysregulated growth and proliferation [23,26]. Additional environmental factors include repeated x-ray exposure, scars from sunburn or disease, and occupational exposure to carcinogens [1,26]. Another important risk factor for development is a family history of melanoma due to similar skin types, shared environmental factors, and genetic predisposition [1,23,26].
The risk of melanoma also changes depending on age, race/ ethnicity, sex, and medical history. Although anyone of any race or ethnicity can develop melanoma, the rates are highest among white patients [1]. Despite the higher incidence in white patients, non-white patients were found to have reduced survival rates [20,21], with the lowest rates in black patients [20]. According to Dawes et.al, black patients had a significantly lower survival rate for stage 1 and 3 melanoma when survival was stratified by race and stage [20]. It is also important to note that ethnic minorities are more likely to present with advanced disease, thicker and deeper melanoma, and melanoma in abnormal locations [20,21]. These findings suggest a disparity in melanoma diagnosis, treatment, and prevention in non-white populations. According to the SEER program, there were more new cases among white males and females than any other racial or ethnic group in the U.S [4]. The risk of melanoma also increases with age. Melanoma is rarely found in young children and adolescents, but the risk increases significantly as age increases [1,23,27]. It is likely that longer exposure to UV radiation and additional environmental factors contribute to the higher incidence rate in the older population [27]. Individuals with inherited conditions such as breast cancer and xeroderma pigmentosum, previous history of cancer, and weakened immune systems are at a higher risk of developing melanoma [1,23].
Surgical
The rationale for surgical treatment
Surgical treatment for metastatic melanoma has had an evolving role in treatment. Historically, surgical treatment options were limited and complete surgical resection was reserved for patients who only had a few metastases to other sites [28]. For patients with widespread metastases, surgery was conventionally not recommended and used mainly for palliation [29]. This idea was centered on the logic that multiple metastases indicated undetectable micrometastases and circulating tumor cells that would lead to the clinical disease shortly after resection, making surgical treatment inconsequential [29]. In short, resection was deemed a local treatment for a systemic disease [28]. However, this idea was investigated by many clinical trials which demonstrated benefit in metastasectomy versus the use of systemic treatment on its own, even before the introduction of more effective systemic therapies [28]. One retrospective study published in 2012 investigated the benefit of surgery alone, surgery followed by systemic therapy, systemic therapy followed by surgery, and systemic therapy alone for patients with stage IV melanoma recurrence [30]. Their findings suggested that more than half of the stage IV patients qualified for resection and exhibited improved survival over patients treated with systemic therapy alone, regardless of the location and amount of metastases. The median overall survival (OS) was 15.8 months for patients who underwent surgery at any time during their treatment, compared to a 6.9-month median OS for those receiving systemic therapy alone [30]. In a phase III clinical trial done by the Southwest Oncology Group in 2011, patients with stage IV melanoma had prolonged OS with complete resection versus systemic treatment alone for resectable melanoma. In those patients, the median relapse-free survival was only 5 months; however, subsequent resections were possible for isolated recurrences [31].
The clinical trials discussed above were done before the introduction of effective immune checkpoint inhibitors and targeted therapies which are increasingly being used as systemic therapies [28]. However, despite the more recent use of effective systemic therapies that can be used in conjunction with surgical resection, there is continued controversy over the part that surgery plays in the treatment of metastatic melanoma. Current National Comprehensive Cancer Network (NCCN) guidelines present that metastatic melanoma is only considered resectable for limited metastatic disease, defined as involving few distant sites, and recommends resection or systemic therapy as the primary treatment [1]. The NCCN also states that widespread melanoma is considered unresectable and not able to fully be treated surgically; however, a surgical approach may be incorporated. With the development and integration of more effective systemic therapies, there continues to be an evolving role between surgical and systemic treatment, even for patients with widespread disease [28,29].
Our current understanding of metastatic disease challenges the earlier idea of circulating tumor cells increasing the disease burden and limiting the benefit of surgical resection. Metastasis is a complex process in which tumor cells must undergo various mutations that allow these cells to not only penetrate the basement membrane but also gain hematogenous access, evade the immune system, and adhere and proliferate in the metastatic site [29,32]. Only a small percent of these cancer cells will be able to achieve all of these processes and generate an organ site-specific metastatic deposit [29]. Therefore, it holds that in a specific patient, various cell populations have and exhibit biologic behavior and an irregular response to systemic treatments, including immunotherapy and targeted therapies [29]. By resecting portions of the patient’s tumor we can decrease the number of cell populations present, including those that may be resistant to systemic treatments [29]. Therefore, it is likely that the remaining cells will respond better to the systemic treatments following surgical resection, jointly with the patient’s immune system [29]. Even partial surgical resection can decrease the immunosuppressive capabilities of tumor deposits [28,29]. This resection also allows for the patient’s immune system to control the residual undetectable metastases and circulating tumor cells [28]. After metastasectomy, there is an increase in the patient's immune response to the melanoma tumor cells, highlighting the importance of the immune system in supporting survival after such metastasectomy [28,33].
Benefits of surgical resection
One of the main benefits of surgical resection in metastatic melanoma is a reduction of the tumor burden which can limit disease progression by disrupting the metastatic cascade and limiting further spread to distant sites [29]. As discussed above, surgical resection also decreases the population diversity of tumor cells which decreases the development of resistance to immunotherapies and may assist in improving the tumor-induced immunosuppression which can limit further disease progression [29,33]. Another benefit is that adverse side effects of surgical resection are generally much better tolerated than those from systemic therapy [29,34]. This is especially true as surgical techniques, anesthesia, and intensive care are improved, bettering outcomes even in extensive surgical resection [34]. Recurrences can also be subsequently treated through secondary resection of metastases [35]. Further, there is increasing support for the combination of surgical resection with systemic therapy; however, delay of metastasectomy can worsen tumor burden and lead to the metastases becoming unresectable [29].
Site-specific surgical treatment
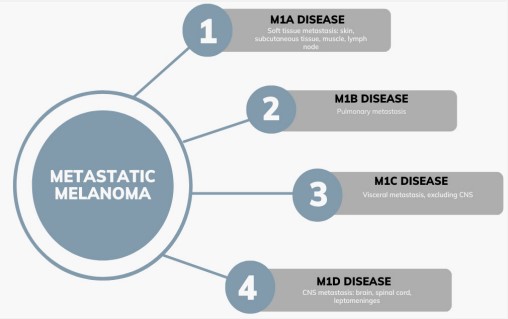
The American Joint Committee on Cancer (AJCC) divides metastatic melanoma into four categories depending on the metastasis site [36]. Patients who had distant metastases to the subcutaneous tissue, skin, muscle or lymph nodes are classified as M1a disease. Patients with metastases to the lung are classified as M1b disease. Patients with metastases to any other visceral site, except the Central Nervous System (CNS), are grouped into M1c disease. M1d disease includes patients who have metastases to any part of the CNS, which includes the brain, spinal cord, and leptomeninges [36]. Patients with M1a disease will have the best survival, followed by those with M1b disease, then M1c disease, and finally the worst prognosis is for those with M1d disease [29]. With surgical resection many factors must carefully be weighed including the serum lactate dehydrogenase, the odds of achieving a complete resection, disease-free interval, tumor doubling time, and response to systemic treatment [28].
M1a disease
Metastases to the soft tissues are among the most likely sites of metastases and typically have improved survival rates compared to other metastatic sites after surgical treatment [37]. The NCCN recommends wide excision for invasive cutaneous melanoma with surgical margins greater than 1 cm to reduce the risk of recurrence. In some studies analyzed by the NCCN, narrower margins were associated with increased local and locoregional recurrence but the results were not consistent across studies [38]. If lymph nodes are involved, typically a complete nodal basin dissection is recommended to aggressively treat the cancer and extend survival [39]. However, surgical morbidity with lymphadenectomy is a risk and it has been shown that up to 12.5% of patients with axillary lymph node dissection and 32.1% of inguinal lymph node dissection have lymphedema following their procedure [40].
M1b disease
M1b disease is defined as a distant spread to the lungs and is the most common site for visceral metastases [40]. For patients with pulmonary metastasis, resection has shown significant benefit and is correlated with increased 5-year survival for 14-35% of patients with resectable disease [40,41]. Factors associated with the overall prognosis for pulmonary metastasis include the number of metastases, time to pulmonary metastases, and completeness of resection [42].
M1c disease
Metastases to other visceral organs, excluding the CNS, are included in M1c disease. This encompasses a wide range of patients; however, similar to other metastatic sites, complete metastasectomy is still associated with improved survival [28]. Patients have a wide range of presentations with M1c disease including bowel obstruction, melena, hematochezia, abdominal pain, or weight loss [28]. The most common site for metastasis in M1c disease is gastrointestinal metastasis [40]. When comparing patients with M1c disease who underwent complete metastasectomy versus those who only received systemic therapy, the median OS was 15 months and 6.3 months, respectively [30]. However, those results include patients with CNS metastases in line with older AJCC guidelines and should be interpreted with caution. Nevertheless, complete metastasectomy is considered to improve median OS for patients with M1c disease.
M1d disease
Despite the worse prognosis, CNS metastases are unfortunately common, especially in patients with existing metastases at other sites [37]. More than half of the patients with metastatic disease at another site will develop CNS metastases, particularly brain metastases [37]. The factors associated with improved survival include solitary lesions, younger age, longer disease-free survival from primary disease to CNS metastasis, no extracranial disease, treatment with surgery and radiotherapy, and good performance status [37].
In the case of CNS involvement, surgical and radiotherapy treatment options include craniotomy or Stereotactic Radiosurgery (SRS), with or without whole-brain radiation therapy (WBRT) [43]. Surgical resection of brain metastases can increase 5-year survival rates in patients with M1d disease with some studies showing an increase in survival rates from 7% to 16% and an extended median OS from 7 to 12 months [43]. However, the decision for surgery is complex due to risks and may only be chosen for large symptomatic tumors due to the risk of hemorrhage [43]. SRS is also widely used and has similar results compared to craniotomy followed by WBRT. This procedure allows for targeted radiation to specific areas and generally works well for brain metastases less than 3.5 cm [43]. However, WBRT has not been shown to have superior efficacy due to melanoma’s relative radioresistance and poses a risk for neurocognitive decline. Despite not showing significant improval in OS, WBRT still has a therapeutic indication for reducing the risk of recurrence at the initial site and distant intracranial sites for patients with multiple intracranial lesions or poor performance status [43].
One comparative study analyzed the variables related to outcomes for patients with cerebral metastasis [44]. This study identified 1137 patients with cerebral metastases who were treated with surgery alone, radiotherapy alone, surgery and postoperative radiotherapy, and palliative care. The patients selected for surgery were based on the amount and sites of metastasis, the presence of extracerebral metastases, and good performance status. Patients who were not candidates for surgery, generally had worse outcomes, with the median survival being 2.1 months for patients receiving palliative care only and 3.4 months for those treated with only radiotherapy. They found that the patients treated with surgery, regardless of the presence of postoperative radiotherapy, had a statistically significant improved survival than those who did not receive surgery [44].
In another retrospective analysis, researchers studied the impact of immunotherapy and targeted therapies with or without surgery and radiotherapy for patients with brain metastases [45]. They found that the median OS was improved when these systemic therapies were combined with surgery and radiosurgery. For immunotherapy specifically, the median OS for immunotherapy with surgery/radiosurgery compared with immunotherapy alone was 25 months and 13 months respectively [45].
Chemotherapy
Chemotherapies for the treatment of metastatic melanoma have long been considered to be ineffective due to the chemoresistant nature of the disease [46]. Despite this fact, cytotoxic chemotherapy has represented the major available therapeutic option for years before the introduction of targeted therapies and immunotherapies [46]. However, even in the age of more efficacious systemic therapies for stage IV melanoma, there may still be a role for chemotherapies [46]. The major chemotherapeutic agents that exhibit some antitumor efficacy in this type of cancer include alkylating agents, platinum analogs, and microtubular toxins, which may be used either alone or in combination [47]. These will be discussed as well as the use and efficacy of a single-agent compared to polychemotherapy for metastatic melanoma.
Single-agent chemotherapy
The most widely used single-agent therapies include dacarbazine, temozolomide, and fotemustine due to their lower toxicity risk and ease of administration [34]. Many more toxic chemotherapeutics have not been shown to have increased survival compared to the agents listed above. The primary therapeutic used for the treatment of this disease is dacarbazine and until its development in the mid-1970s, there were no therapeutic agents that exhibited true efficacy against metastatic melanoma [48]. Dacarbazine is an alkylating agent that induces damage to DNA by adding a methyl group to the guanine base in the O6 position, which is thought to induce apoptosis [49]. It includes the adverse effects of myelosuppression, fatigue, and mild nausea and vomiting but most patients can maintain their quality of life [49]. However, even single-agent dacarbazine has limited efficacy and has been shown to only have an objective response rate (ORR) of 15.3% in a pooled analysis of 23 randomized, controlled trials[47]. In this study, those responses were often not long-lasting with less than 2% of patients alive at the 6-year mark [47]. However, despite limited efficacy it remains in use today and until the introduction of targeted therapies, it constituted the standard of care treatment [50]. Many studies have also analyzed the efficacy of dacarbazine’s orally administered analog, temozolomide. Temozolomide not only has improved oral bioavailability but also can penetrate the CNS which makes it an enticing treatment for melanoma brain metastases [47]. Several phase III randomized clinical trials have investigated the median OS and ORR for patients treated with dacarbazine and temozolomide. In these studies, no significant difference was found between the two in median OS, progression-free survival, or ORR [51,52]. Therefore, the decision between the two is typically based on the cost, route of administration, and the presence of metastases to the brain [47].
Other conventional single-agent chemotherapeutic options include microtubular assembly inhibitors, such as vindesine, vinblastine, and paclitaxel, which have been shown to have moderate single-agent activity for treating metastatic melanoma [48]. Platinum analogs, such as cisplatin, and nitrosoureas, such as carmustine and fotemustine, have also been used for single-agent chemotherapy for metastatic melanoma. Cisplatin and carboplatin have moderate activity and efficacy, while nitrosoureas have activity comparable to dacarbazine [47]. When compared with dacarbazine in a phase III clinical trial involving metastatic melanoma patients, fotemustine was associated with higher ORR and improved survival, with the survival being 7.3 and 5.6 months respectively [53].However, nitrosoureas are thought to cause more adverse effects, including myelosuppression and alopecia [47].
Polychemotherapy
Different combinations of the therapies mentioned above have been proposed to work synergistically with each other for the treatment of metastatic melanoma. Several studies have been done to investigate this idea and many have demonstrated slightly improved outcomes for patients, however, polychemotherapy regimens are also associated with higher toxicities [47]. One regimen, known as the Dartmouth regimen, is a combination of cisplatin, dacarbazine, carmustine, and tamoxifen originally reported in a phase III randomized clinical trial to have an ORR of 55% [54]. However, despite this promising result, when compared to single-agent dacarbazine, it did not translate to significant improvement in survival for patients, and myelosuppression, fatigue, nausea, and vomiting were higher in those receiving the combination regimen [54]. Many of the other combination regimens that have been investigated and compared to single-agent dacarbazine have only shown some improvement in tumor response rate but have not been associated with significantly prolonged survival [50,55]. These include combinations such as cisplatin, vinblastine, and dacarbazine (CVD) regimen, which was eventually used as a framework for later biochemotherapy regimens combining IL-2 and interferons [47,56]. Other combinations include paclitaxel and carboplatin (PC) which showed some antitumor effect, especially as a second-line treatment for patients who had previously received chemotherapy [57].
Chemotherapy for CNS metastasis
As mentioned above, there are limitations to cytotoxic chemotherapy for melanoma metastases, particularly to the CNS. The therapies that have shown the most benefit because of their ability to cross the blood-brain barrier are fotemustine and temozolomide [58]. In one phase II trial fotemustine had a response rate of 25% for patients with brain metastases; however, a phase III trial showed fotemustine to only have a response rate of 5.9% when compared with dacarbazine. Temozolomide performs similarly with one trial showing a response rate of 6% for patients with brain metastases [58]. The risk of toxicity is the biggest concern when combining cytotoxic chemotherapy with other procedures used to treat brain metastases such as WBRT and SRS [58]. Some studies have shown that the additive benefit of those therapies is not significant and poses much higher toxicity to the patients [58].
Future directions for chemotherapies
Despite the limitations of chemotherapies for metastatic melanoma, there have been many hypotheses about the benefit of combining cytotoxic chemotherapy with newer targeted therapies and immunotherapies in specific subgroups of patients [46]. This may be indicated for patients with specific genetic and molecular features or who have not responded to targeted therapies or immune checkpoint inhibitors alone. There are many exciting applications for cytotoxic therapies in conjunction with newer treatment options that are currently being studied and pose an interesting future for the treatment of metastatic melanoma [46].
Immune checkpoint inhibitors
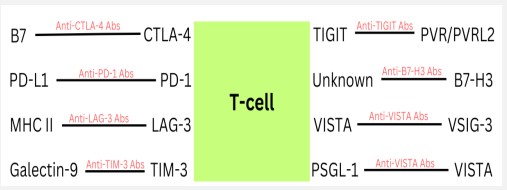
ICIs are a revolutionary immuno-oncology innovation that ended a stalemate in developing new melanoma treatments [59-61]. One crucial step in activating the immune system against melanoma is the activation of T-cells [62]. Melanoma cells have mechanisms of evading this immunosurveillance and therefore suppressing an immune response [60,62]. T-cells have two inhibitory molecules, cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed T-cell death 1 (PD-1) receptor, that downregulate T-cell activation when they interact with their ligand [62]. CTLA-4 competes with CD28 for B7 binding on antigen-presenting cells (APCs) with increased affinity and interrupts this co-stimulatory signal, leading to T-cell anergy [62]. Melanoma can upregulate CTLA-4 in T-cells, leading to a lack of T-cell activation, which downregulates the response of the immune system [62,63]. Additionally, melanoma cells can express PD-L1 which can interact with PD-1 receptors on activated Tcells and decrease T-cell function [59]. ICIs interrupt these inhibitory signaling pathways and restore the antitumor action of T-cells [60].
Anti-CTLA-4 antibodies
Ipilimumab, the first ICI to become FDA approved in 2011, is a human IgG1 monoclonal antibody that blocks the CTLA-4 receptor and allows for T-cell activation to eliminate melanoma tumor cells [61,64,65]. Phase III clinical trials (NCT00094653) showed a significant increase in OS rates of patients with metastatic melanoma that have tried previous treatments when given ipilimumab treatment [61]. Patients who received ipilimumab alone had a median survival rate of 10.1 months, while the control group, who received only a glycoprotein 100 (gp100) peptide vaccine, had a median survival rate of 6.4 months [64]. Patients who received ipilimumab plus gp100 had a median OS of 10.0 months, while the control group had a median survival rate of 6.4 months [64]. There was no statistical difference reported between the two groups treated with ipilimumab [64]. An additional clinical trial (NCT00324155) showed that the treatment with ipilimumab and dacarbazine, a chemotherapy, had improved outcomes in patients with untreated metastatic melanoma when compared to treatment with dacarbazine alone [61,66]. The OS of the patients receiving ipilimumab plus dacarbazine was 11.2 months, while the OS of patients receiving dacarbazine plus placebo was 9.1 months [66]. Additionally, patients receiving both ipilimumab and dacarbazine showed higher survival rates at years 1 through 3 than the patients receiving dacarbazine plus placebo [66].
Anti-PD-1 antibodies
Pembrolizumab, the first FDA-approved PD-1 treatment of metastatic melanoma, is a humanized IgG4-kappa monoclonal antibody that interacts with the PD-1 receptor to decrease immune response suppression [61,67]. A phase III clinical trial (NCT01866319) revealed that the estimated six-month progression-free-survival rate of patients who had advanced melanoma treated with pembrolizumab every two weeks was 47.3%, while patients treated with ipilimumab had an estimated six-month progression-free-survival rate of 26.5% [68]. Additionally, this trial showed a higher 12-month OS rate for patients treated with pembrolizumab as compared to ipilimumab treatment [65]. Another anti-PD-1 FDA-approved treatment is nivolumab, which is a human IgG4 monoclonal antibody that also blocks the interaction of the PD-1 receptor with its ligand [65]. In a phase III clinical trial (NCT01844505), patients treated with nivolumab had an OS rate at five years of 44%, while patients treated with ipilimumab had an OS rate at five years of 26% [69]. Furthermore, clinical trials have shown that anti-PD-1 antibodies have less toxic effects when compared to ipilimumab [59]. Other anti-PD-1 antibody treatments that have been studied include atezolizumab, durvalumab, cemiplimab, avelumab, and spartlizumab [70].
Anti-CTLA-4 and PD-1 combinations
ICI treatment combinations of anti-CTLA-4 and anti-PD-1 antibodies have been investigated to treat metastatic melanoma. In a phase III clinical trial (NCT01844505), a combination treatment of ipilimumab plus nivolumab has an OS rate at 5 years of 52%, whereas patients treated with only nivolumab had an OS rate of 44% and patients treated with only ipilimumab had an OS rate of 26% [69].
Limitations
ICIs are a revolutionary discovery in the world of immuno-oncology; however, they do not come without limitations. Tumor cells are able to develop resistance to ICIs through mechanisms of additive tumor mutations over time [61]. These mutations may remove tumor-specific antigens, leading to the inability of T-cells to activate, target, and damage tumor cells [61]. Patients have also been observed to have β2-microglobulin, an important protein in MHC Class I, mutations in melanoma tumor cells, tied to anti-PD-1 treatment failure [61]. Research has also highlighted mutations in mechanisms that signal T-cells to the site of the melanoma cells, leading to ICI resistance [61]. Besides resistance, ICIs have only been shown effective for a subset of cancer patients and are linked with a variety of adverse immune-related events [61,71]. For patients receiving ipilimumab treatment, common adverse immune-related events occur in the skin and gastrointestinal tract [61]. For patients receiving anti-PD-1 treatment, common adverse immune-related events included fatigue, fever, chills, rash, diarrhea, endocrine toxicities, hepatic toxicities, and pneumonitis [72]. Researchers are working to describe various biomarkers of patients to determine who may most benefit from treatment with ICIs [61].
Novel immune checkpoint inhibitors
Anti-LAG-3 antibodies
CTLA-4 and PD-1 have been in the spotlight as the major targets of ICIs; however, there are other immune checkpoints of interest. Lymphocyte activation gene-3 (LAG-3) can be present on activated T-cells and bind to MHC Class II with an increased affinity compared to CD4 [73]. This binding decreases T-cell signal transduction, due to its association with CD3, leading to a downregulation of T-cell response and T-cell proliferation [73,74]. LAG-3 is upregulated in melanoma and is often co-expressed with PD-1 [75]. Relatlimab is the first human IgG4 antibody that can bind LAG-3 and restore the action of effector T-cells [76]. In a double-blinded, phase II-III clinical trial (NCT03470922), the treatment of melanoma with relatlimab plus nivolumab had a median progression-free survival of 10.1 months, and treatment with only nivolumab had a median progression-free survival of 4.6 months, showing a clear increase in progression-free survival with the addition of the LAG-3 inhibitor [76].
Anti-TIM-3 antibodies
T-cell immunoglobulin-3 (TIM-3) is a receptor expressed on the surface of CD4+ Th1 cells, CD8+ T-cells, regulatory T-cells (Tregs), and other innate immune cells [77]. TIM-3 binding by its ligand, galectin-9, can decrease CD8+ T-cell activity and potentially recruit immune suppression cells such as Tregs [74]. TIM3 expression has been shown to be increased in patients with metastatic melanoma [77]. Additionally, in preclinical studies, anti-TIM-3 treatments have been shown to be synergistic with anti-PD-1 treatments for patients with advanced melanoma [78]. BGB-A425 is a humanized IgG1-variant monoclonal antibody that can bind TIM-3, which is currently undergoing investigation in a phase I/II clinical trial (NCT03744468) where they hope to determine safety, recommended dose, and antitumor effects of the treatment [79]. Patients receive the BGB-A425 treatment along with either an anti-PD-1 monoclonal antibody or along with an anti-PD-1 monoclonal antibody and an antiLAG-3 monoclonal antibody [76].
Anti-TIGIT antibodies
T-cell immunoglobulin and ITIM domain (TIGIT) is an inhibitory receptor in the CD28 family and is a marker of exhausted CD8+ T-cells and Tregs in tumor microenvironments [74]. TIGIT competes with CD226 and CD96 on T-cells for the ligands PVR and PVRL2, similar to the pathway of CD28, CTLA-4, and B7 [74]. TIGIT interaction with PVR downregulates the action of T-cells and causes upregulated IL-10 production, decreasing the immune response of T-cells [74]. Research has shown that the PVR ligand is upregulated in melanoma [81]. Tiragolumab is a human IgG1-kappa anti-TIGIT monoclonal antibody that can block the interaction of TIGIT with its ligand [82]. Genentech is leading the development of tiragolumab, the most promising anti-TIGIT antibody treatment, which is currently undergoing phase II clinical trial as an experimental treatment for anti-PD-1 antibodyresistant metastatic melanoma (NCT05483400) [73,83,84].
Anti-B7-H3 antibodies
B7-H3 (CD276) is a cell surface molecule that is a part of the B7 family that has a higher expression on APCs and malignant tumors [85]. The receptor for B7-H3 is currently unknown, but it has been shown to have immune and T-cell suppression activity, protecting against CD8+ T-cells [85,86]. Additional preclinical studies revealed that inhibiting B7-H3 expression in metastatic melanoma reduced the growth of the melanoma cells and increased their sensitivity to chemotherapy and other target treatments [87]. Murine models have shown a synergistic antitumor effect between anti-PD-1 and anti-B7-H3 monoclonal antibodies [88]. Enoblituzumab is a humanized anti-B7-H3 monoclonal antibody and was well tolerated in a phase I clinical trial (NCT02475213) where it was administered along with pembrolizumab [85].
Anti-VISTA antibodies
VISTA is expressed on resting T-cells, is structurally similar to PD-1, and can suppress T-cell response to cancer [70,89]. It is usually expressed on hematopoietic cells; however, there have been reports of VISTA’s expression in melanoma cell lines and patient samples [70]. VISTA can interact with both V-set and Ig domain-containing 3 (VSIG-3) and P-selectin glycoprotein ligand 1 (PSGL-1) [90]. VISTA has been shown to promote tumor onset and upregulate the expression of PD-L1 on macrophages that would infiltrate the tumor [91]. CI-8993 is a human IgG1-kappa monoclonal antibody against the VISTA ligand that is presently undergoing phase I clinical trial (NCT04475523) for patients with advanced solid tumor malignancies [92].
Preclinical investigations
PD-1/PD-L1 small molecule inhibitors
Currently, FDA-approved PD-1 ICIs are limited to monoclonal antibodies [93]. However, monoclonal antibodies are expensive to produce and have limitations such as the risk of immunogenicity, bioavailability issues, and poor tissue or biological barrier penetration [93,94]. One way to circumvent these issues is with the production of small molecules that can also block the interaction between PD-1 and PD-L1 [93]. A handful of PD-1 modulating small molecules have been developed, but the development of these molecules is in its inception and much preclinical work still needs to be done [93]. CA-170 is an orally available small molecule that has been shown to have activity against PD-1 and VISTA signaling [95]. Preclinical research has shown that CA-170 does not prevent the binding between PD-1 receptors and PD-L1, but leads to this ternary binding complex's inactivation [95]. Preclinical models have also shown CA-170 to have activity against tumor growth [95]. CA-170 did undergo a phase I clinical trial (NCT02812875) which showed that the molecule was generally safe and plasma levels proportional to the dose [95,96]. Another small molecule, MAX-10181, is an orally bioavailable PD-L1 inhibitor and studies have shown it has similar efficacy to durvalumab, a PD-L1 antibody treatment [94]. MAX-10181 is currently undergoing a phase I clinical trial (NCT04122339) [94,97]. There have been other PD-1 and PD-L1 modulating small molecules disclosed from Harvard, Aurigene, Augusta University, Bristol-Myers Squibb, Incyte, and Polaris [93]. Recently, there was a study identifying N-{4-[(4-chlorobenzyl)oxy]benzyl}N-(4-pyridinylmethyl)amine (CBPA) as a new small molecule PD-L1 inhibitor [94]. Using primary tumor models, this study showed CBPA’s ability to increase CD8+ T-cell infiltration and cytokine secretion, highlighting its potential antitumor actions in melanoma treatment [94].
Photodynamic therapy
Photodynamic therapy (PDT) has been researched as a unique cancer treatment and has been used as a treatment alternative for patients with non-melanoma skin cancer [98,99]. PDT involves a light-sensitive, photosensitizer (PS) drug targeted to cancer cells that is then excited by a specific wavelength of light [98,100]. In the presence of oxygen, the excited PS drug will produce Reactive Oxygen Species (ROS), which encourages oxidative stress to the cancer cells, with the hope this induces apoptosis, autophagy, or necrosis [98,100]. Limitations of PDT exist, such as toxic side effects, skin photosensitivity, and poor tumor localization [100]. Preclinical researchers are working on better options for drug delivery of these photodynamic treatments, which may help overcome these limitations and make PDT a more viable option for treating metastatic melanoma. PDT is worthy of preclinical research as it has been shown to encourage the regression of metastatic malignancies[99]. A recent study discussed Chlorin e6 (Ce6) as a potential PDT that has sufficient antitumor activity [99]. It has a short half-life, which encourages quick clearance, limiting potential toxic side effects [99]. The study utilized a mice model and melanoma cells (B16F10), and Ce6-PDT showed significant tumor shrinkage [99]. A limitation of PDT is the ability for the light to penetrate deep into the tissues; however, there is current research on how to circumvent this issue and increase tissue penetrations using methods such as fiber optics, microendoscopy, and upconverting nanoparticles [101].
Nanotechnology for drug delivery
For treatments like PDT and many other cancer treatments, targeted delivery to tumors is crucial to maximize efficacy and minimize unwanted, toxic side effects. A promising area of research in the drug delivery space is the use of nanotechnology, such as nanoparticles (NPs), to target treatments to their desired locations. Current treatments for melanoma may have poor bioavailability, low water solubility, or rapid clearance or metabolism, limiting their effectiveness [102]. With targeted drug delivery systems using nanotechnology, researchers would potentially be able to circumvent these limitations of current metastatic melanoma treatments [102]. NPs have two types of targeting strategies: passive and active. Passive targeting involves modulating NPs for better uptake, permeability, or retention in the desired location [102]. Active targeting involves modulating NPs to have specific recognition of tumor-specific antigens using various proteins or antibodies [102]. Types of NPs being studied as melanoma treatment drug delivery systems include liposomes, inorganic NPs, polymer NPs, and natural nanosystems, like exosomes, for example [102]. Using NPs specifically with ICIs offers a solution to overcome their various limitations. There have been a handful of NP formulations using polymers such as poly(ethylene glycol) (PEG), poly(D, L-lactide) (PLA), and poly(lactic-co-glycolic acid) (PLGA) studied due to their biocompatibility and ability to be degraded safely [70]. A recent preclinical study highlighted a potential new NP delivery system for targeting and treating melanoma, an L-Cysteine (LCys)-coated superparamagnetic iron oxide NP (SPION) loaded with doxorubicin, a chemotherapy [103]. This NP system had good melanoma cell internalization using both human (A375) and mouse (B16F10) model melanoma cell lines [103]. This study did show apoptosis of melanoma cells treated with the NP system; however, further in vivo animal studies will need to be completed in order to determine the NPs in vivo targeting ability [103].
Conclusion
Melanoma is a serious type of skin cancer that affects 100,000 Americans yearly and makes up 5% of all new cancer cases per year [4]. Metastatic melanoma, in specific, accounts for 1.3% of all cancer deaths yearly, highlighting its impact on the health system [24]. Melanoma can metastasize to other parts of the body and has a tropism for metastasizing to the brain [2,9]. Due to recent and revolutionary innovations, there are a variety of treatment options for melanoma, including surgery, chemotherapy, radiotherapy, and ICIs. Surgery cannot treat widespread metastatic melanoma entirely; however, studies have shown that surgical resection improves patient outcomes, regardless of the number of metastases or where they are located in the body [28-30]. Many chemotherapies have proved unsuccessful in the treatment of metastatic melanoma; however, dacarbazine has shown antitumor efficacy and is the primary chemotherapy used in treatment [46,48]. Researchers are now exploring ways to use chemotherapies in conjunction with newer treatment options, such as ICIs, to offer more treatment options for patients [46]. Classical ICIs, like anti-CTLA-4 and anti-PD-1 antibodies, offer a subset of metastatic melanoma patients with innovative treatment options; however, there is still work to be done to improve ICIs for better patient outcomes [61]. Novel ICIs undergoing preclinical research and early-phase clinical trials include anti-LAG-3, anti-TIM-3, antiTIGIT, anti-B7-H3, and anti-VISTA antibodies, with the hope that new effective ICIs can be discovered. The preclinical space has exciting new research for possible alternatives and additions to metastatic melanoma treatment, including small molecule inhibitors instead of monoclonal antibodies, photodynamic therapy, and nanotechnology. In conclusion, there are limitations to the current treatments available for metastatic melanoma; however, the ongoing preclinical and clinical investigations offer exciting innovations for the future.
References
- NCCN Guidelines for Patients Melanoma. 2021.
- Braeuer RR, Watson IR, Wu CJ, Mobley AK, Kamiya T, et al. Why is melanoma so metastatic? Pigment Cell Melanoma Res. 2014;27(1):19-36.
- Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019; 20: 1366-1379.
- Melanoma of the Skin - Cancer Stat Facts. SEER. 2023.
- Sardana K, Chakravarty P, Goel K. Optimal management of common acquired melanocytic nevi (moles): current perspectives. Clin Cosmet Investig Dermatol. 2014; 7: 89-103.
- Liu Y, Sheikh MS. Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol Cell Pharmacol. 2014; 6: 228.
- Elder D. Melanoma progression | Elsevier Enhanced Reader.
- Damsky WE, Theodosakis N, Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014; 33: 2413-2422.
- Damsky WE, Rosenbaum LE, Bosenberg M. Decoding Melanoma Metastasis. Cancers. 2010; 3: 126-163.
- Damsky W, Bosenberg M. Melanocytic nevi and melanoma: unraveling a complex relationship. Oncogene. 2017; 36: 5771-5792.
- Frischhut N, Zelger B, Andre F, Zelger BG. The spectrum of melanocytic nevi and their clinical implications. J Dtsch Dermatol Ges. 2022; 20: 483-504.
- Goldstein AM, Tucker MA. Dysplastic Nevi and Melanoma. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2013; 22: 528-532.
- Balu M, Kelly KM, Zachary CB, Harris RM, Krasieva TB, et al. Distinguishing between benign and malignant melanocytic nevi by in vivo multiphoton microscopy. Cancer Res. 2014; 74: 2688-2697.
- Stefanato CM. The “Dysplastic Nevus” Conundrum: A Look Back, a Peek Forward. Dermatopathology. 2018; 5: 53-57.
- Reddy KK, Farber MJ, Bhawan J, Geronemus RG, Rogers GS. Atypical (Dysplastic) Nevi: Outcomes of Surgical Excision and Association With Melanoma. JAMA Dermatol. 2013; 149: 928-934.
- Urso C. Are growth phases exclusive to cutaneous melanoma? J Clin Pathol. 2004; 57: 560.
- Crowson AN, Magro CM, Mihm MC. Prognosticators of melanoma, the melanoma report, and the sentinel lymph node. Mod Pathol. 2006; 19: S71-S87.
- Adler NR, Haydon A, McLean CA, Kelly JW, Mar VJ. Metastatic pathways in patients with cutaneous melanoma. Pigment Cell Melanoma Res. 2017; 30: 13-27.
- Javid MH. Melanoma skin cancer dataset of 10000 images. 2022.
- Dawes SM, Tsai S, Gittleman H, Barnholtz-Sloan JS, Bordeaux JS. Racial disparities in melanoma survival | Elsevier Enhanced Reader.
- Mahendraraj K, Sidhu K, Lau CSM, McRoy GJ, Chamberlain RS, Smith FO. Malignant Melanoma in African–Americans. Medicine (Baltimore). 2017; 96: e6258.
- Braeuer RR, Watson IR, Wu CJ, Mobley AK, Kamiya T, et al. Why is melanoma so metastatic? Pigment Cell Melanoma Res. 2014; 27: 19-36.
- Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: Epidemiology, Risk Factors, Pathogenesis, Diagnosis and Classification. In Vivo. 2014.
- Sundararajan S, Thida AM, Yadlapati S, Koya S. Metastatic Melanoma. StatPearls Publishing; 2022.
- Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. NeuroOncol. 2017; 19: 1511-1521.
- Volkovova K, Bilanicova D, Bartonova A, Letašiová S, Dusinska M. Associations between environmental factors and incidence of cutaneous melanoma. Review. Environ Health. 2012; 11: S12.
- Paulson KG, Gupta D, Kim TS, Veatch JR, Byrd DR, et al. Age-Specific Incidence of Melanoma in the United States. JAMA Dermatol. 2020; 156: 57-64.
- Enomoto LM, Levine EA, Shen P, Votanopoulos KI. Role of Surgery for Metastatic Melanoma. Surg Clin North Am. 2020; 100: 127-139.
- Leung AM, Hari DM, Morton DL. Surgery for distant melanoma metastasis. Cancer J Sudbury Mass. 2012; 18: 176-184.
- Howard JH, Thompson JF, Mozzillo N, Nieweg OE, Hoekstra HJ, et al. Metastasectomy for distant metastatic melanoma: analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I). Ann Surg Oncol. 2012; 19: 2547-2555.
- Sosman JA, Moon J, Tuthill RJ, Warneke JA, Vetto JT, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430. Cancer. 2011; 117: 4740-4706.
- Zetter BR. The cellular basis of site-specific tumor metastasis. N Engl J Med. 1990; 322: 605-612.
- Hsueh EC, Gupta RK, Yee R, Leopoldo ZC, Qi K, et al. Does endogenous immune response determine the outcome of surgical therapy for metastatic melanoma? Ann Surg Oncol. 2000; 7: 232-238.
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet Lond Engl. 2005; 365: 687-701.
- Ollila DW, Hsueh EC, Stern SL, Morton DL. Metastasectomy for recurrent stage IV melanoma. J Surg Oncol. 1999; 71: 209-213.
- Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67: 472-492.
- Martinez SR, Young SE. A rational surgical approach to the treatment of distant melanoma metastases. Cancer Treat Rev. 2008; 34: 614-620.
- Swetter SM, Thompson JA, Albertini MR, Barker CA, Baumgartner J, et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2021. J Natl Compr Cancer Netw JNCCN. 2021; 19: 364-376.
- Lasithiotakis K, Zoras O. Metastasectomy in cutaneous melanoma. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2017; 43: 572-580.
- Raigani S, Cohen S, Boland GM. The Role of Surgery for Melanoma in an Era of Effective Systemic Therapy. Curr Oncol Rep. 2017; 19: 17.
- Tafra L, Dale PS, Wanek LA, Ramming KP, Morton DL. Resection and adjuvant immunotherapy for melanoma metastatic to the lung and thorax. J Thorac Cardiovasc Surg. 1995; 110: 119-128; discussion 129.
- Leo F, Cagini L, Rocmans P, et al. Lung metastases from melanoma: when is surgical treatment warranted? Br J Cancer. 2000; 83: 569-572.
- Nicholas S, Mathios D, Jackson C, Lim M. Metastatic melanoma to the brain: surgery and radiation is still the standard of care. Curr Treat Options Oncol. 2013; 14: 264-279.
- Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol Off J Am Soc Clin Oncol. 2004; 22: 1293-1300.
- Amaral T, Tampouri I, Eigentler T, Keim U, Klumpp B, et al. Immunotherapy plus surgery/radiosurgery is associated with favorable survival in patients with melanoma brain metastasis. Immunotherapy. 2019; 11: 297-309.
- Simon A, Kourie HR, Kerger J. Is there still a role for cytotoxic chemotherapy after targeted therapy and immunotherapy in metastatic melanoma? A case report and literature review. Chin J Cancer. 2017; 36: 10.
- Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncol Williston Park N. 2009; 23: 488-496.
- Wick MR, Gru AA. Metastatic melanoma: Pathologic characterization, current treatment, and complications of therapy. Semin Diagn Pathol. 2016; 33: 204-218.
- National Toxicology Program. Dacarbazine. Rep Carcinog Carcinog Profiles. 2011; 12: 127-128.
- Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018; 2: CD011123.
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2000; 18: 158-166.
- Patel PM, Suciu S, Mortier L, Kruit WH, Robert C, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer Oxf Engl 1990. 2011; 47: 1476-1483.
- Avril MF, Aamdal S, Grob JJ, Hauschild A, Mohr P, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol Off J Am Soc Clin Oncol. 2004; 22: 1118-1125.
- Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 1999; 17: 2745-2751.
- Ridolfi R, Chiarion-Sileni V, Guida M, Romanini A, Labianca R, et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. J Clin Oncol Off J Am Soc Clin Oncol. 2002; 20: 1600-1607.
- Legha SS, Ring S, Papadopoulos N, Plager C, Chawla S, Benjamin R. A prospective evaluation of a triple-drug regimen containing cisplatin, vinblastine, and dacarbazine (CVD) for metastatic melanoma. Cancer. 1989; 64: 2024-2029.
- Rao RD, Holtan SG, Ingle JN, Croghan GA, Kottschade LA, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006; 106: 375-382.
- Long GV, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma: the emerging role of systemic therapies. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Annu Meet. 2013; 393-398.
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. The Lancet. 2021; 398: 1002-1014.
- Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018; 50: 165.
- Singh S, Numan A, Agrawal N, Tambuwala MM, Singh V, Kesharwani P. Role of immune checkpoint inhibitors in the revolutionization of advanced melanoma care. Int Immunopharmacol. 2020; 83: 106417.
- Mahmoud F, Shields B, Makhoul I, Avaritt N, Wong HK, et al. Immune surveillance in melanoma: From immune attack to melanoma escape and even counterattack. Cancer Biol Ther. 2017; 18: 451-469.
- Hui E. Immune checkpoint inhibitors. J Cell Biol. 2019; 218: 740-741.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010; 363: 711-723.
- Sood S, Jayachandiran R, Pandey S. Current Advancements and Novel Strategies in the Treatment of Metastatic Melanoma. Integr Cancer Ther. 2021; 20: 1534735421990078.
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011; 364: 2517-2526.
- Hamid O, Robert C, Daud A, Stephen Hodi F, Hwu WJ, et al. Safety and Tumor Responses with Lambrolizumab (Anti–PD-1) in Melanoma. N Engl J Med. 2013; 369: 134-144.
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015; 372: 2521-2532.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019; 381: 1535-1546.
- Mahdavi Gorabi A, Sadat Ravari M, Sanaei MJ, Davaran S, Kesharwani P, et al. Immune checkpoint blockade in melanoma: Advantages, shortcomings and emerging roles of the nanoparticles. Int Immunopharmacol. 2022; 113: 109300.
- Immune-Related Adverse Events Associated with Immune Checkpoint Blockade | NEJM. 2023.
- Naidoo J, Page DB, Li BT, Connell LC, Schindler K, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015; 26: 2375-2391.
- Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, et al. MHC Class II Engagement by Its Ligand LAG-3 (CD223) Contributes to Melanoma Resistance to Apoptosis. J Immunol. 2011; 186: 5173-5183.
- Bhandaru M, Rotte A. Monoclonal Antibodies for the Treatment of Melanoma: Present and Future Strategies. In: Steinitz M, ed. Human Monoclonal Antibodies: Methods and Protocols. Methods in Molecular Biology. Springer, 2019; 83-108.
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012; 72: 917-927.
- Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med. 2022; 386: 24-34.
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016; 44: 989-1004.
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen–specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010; 207: 2175-2186.
- Desai J, Meniawy T, Beagle B, Li Z, Mu S, et al. Bgb-A425, an investigational anti-TIM-3 monoclonal antibody, in combination with tislelizumab, an anti-PD-1 monoclonal antibody, in patients with advanced solid tumors: A phase I/II trial in progress. J Clin Oncol. 2020; 38: TPS3146-TPS3146.
- BeiGene. Phase 1-2 Study Investigating Safety, Tolerability, Pharmacokinetics and Preliminary Antitumor Activity of Various Combinations of BGB-A425 and LBL-007 With Tislelizumab in Patients With Advanced Solid Tumors. clinicaltrials.gov; 2022.
- Inozume T, Yaguchi T, Furuta J, Harada K, Kawakami Y, Shimada S. Melanoma Cells Control Antimelanoma CTL Responses via Interaction between TIGIT and CD155 in the Effector Phase. J Invest Dermatol. 2016; 136: 255-263.
- Cho BC, Abreu DR, Hussein M, Cobo M, Patel AJ, et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a firstline treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022; 23: 781-792.
- Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018; 29: 71-83.
- University Medical Center Groningen. Open Label Phase 2 Basket Trial With Atezolizumab and Tiragolumab in Solid Tumors: TIRACAN. clinicaltrials.gov; 2022.
- Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer. 2022; 10: e004424.
- Prasad DVR, Nguyen T, Li Z, Yang Y, Duong J, et al. Murine B7-H3 Is a Negative Regulator of T Cells1. J Immunol. 2004; 173: 2500-2506.
- Flem-Karlsen K, Tekle C, Andersson Y, Flatmark K, Fodstad Ø, et al. Immunoregulatory protein B7-H3 promotes growth and decreases sensitivity to therapy in metastatic melanoma cells. Pigment Cell Melanoma Res. 2017; 30: 467-476.
- Yonesaka K, Haratani K, Takamura S, Sakai H, Kato R, et al. B7-H3 Negatively Modulates CTL-Mediated Cancer Immunity. Clin Cancer Res. 2018; 24: 2653-2664.
- Lee JB, Ha SJ, Kim HR. Clinical Insights Into Novel Immune Checkpoint Inhibitors. Front Pharmacol. 2021; 12: 681320.
- Yuan L, Tatineni J, Mahoney KM, Freeman GJ. VISTA: A Mediator of Quiescence and a Promising Target in Cancer Immunotherapy. Trends Immunol. 2021; 42: 209-227.
- Rosenbaum SR, Knecht M, Mollaee M, Zhong Z, Erkes DA, et al. FOXD3 Regulates VISTA Expression in Melanoma. Cell Rep. 2020; 30: 510-524.e6.
- Curis, Inc. Phase 1 Study of CI-8993 Anti-VISTA Antibody in Patients With Advanced Solid Tumor Malignancies. clinicaltrials. gov; 2022.
- Jiao P, Geng Q, Jin P, Su G, Teng H, et al. Small Molecules as PD-1/ PD-L1 Pathway Modulators for Cancer Immunotherapy. Curr Pharm Des. 24: 4911-4920.
- Wang F, Ye W, He Y, Zhong H, Zhu Y, et al. Identification of CBPA as a New Inhibitor of PD-1/PD-L1 Interaction. Int J Mol Sci. 2023; 24: 3971.
- Sasikumar PG, Sudarshan NS, Adurthi S, Ramachandra RK, Samiulla DS, et al. PD-1 derived CA-170 is an oral immune checkpoint inhibitor that exhibits preclinical anti-tumor efficacy. Commun Biol. 2021; 4: 1-12.
- Curis, Inc. A Phase 1, Open-Label, Dose Escalation and Dose Expansion Trial Evaluating the Safety, Pharmacokinetics, Pharmacodynamics, and Clinical Effects of Orally Administered CA-170 in Patients With Advanced Tumors and Lymphomas. clinicaltrials.gov; 2020.
- Maxinovel Pty., Ltd. A Phase I Study of MAX-10181 Given Orally to Patients With Advanced Solid Tumor. clinicaltrials.gov; 2022.
- Naidoo C, Kruger CA, Abrahamse H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol Cancer Res Treat. 2018; 17: 1533033818791795.
- Shrestha R, Mallik SK, Lim J, Gurung P, Magar TBT, Kim YW. Efficient Synthesis of Chlorin e6 and Its Potential Photodynamic Immunotherapy in Mouse Melanoma by the Abscopal Effect. Int J Mol Sci. 2023; 24: 3901.
- Gunaydin G, Gedik ME, Ayan S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front Chem. 2021; 9.
- Mallidi S, Anbil S, Bulin AL, Obaid G, Ichikawa M, Hasan T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics. 2016; 6: 2458-2487.
- Zeng H, Li J, Hou K, Wu Y, Chen H, Ning Z. Melanoma and Nanotechnology-Based Treatment. Front Oncol. 2022; 12.
- Toderascu LI, Sima LE, Orobeti S, Florian PE, Icriverzi M, et al. Synthesis and Anti-Melanoma Activity of L-Cysteine-Coated Iron Oxide Nanoparticles Loaded with Doxorubicin. Nanomaterials. 2023; 13: 621.