Review article
Volume 2, Issue 4
Advancing Analytics of EEG Signals
Fnu Ruchika*; Durga Neupane; Siddharth Shah; Maliya Delawan; Brandon Lucke-Wold
Department of Neurosurgery, University of Florida, USA.
Corresponding Author:
Fnu Ruchika
Email: Ruchikadr97@gmail.com
Received : Feb 14, 2023 Accepted : Apr 05, 2023 Published Online : Apr 12, 2023
Citation: Ruchika F, Neupane D, Shah S, Delawan M, Lucke-Wold B, et al. Advancing Analytics of EEG Signals. Med Discoveries. 2023; 2(4): 1031.
Copyright: © 2023 Ruchika F. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Electro Encephalo Graphy (EEG) is a non-invasive diagnostic tool that is widely used in the field of neurosurgery. The EEG measures the electrical activity of the brain, which provides essential information about brain function and can help diagnose various neurological conditions. In neurosurgery, EEG monitors the brain during surgery to ensure that the patient’s brain function remains stable and minimize the risk of neurological complications. EEG is also used in the preoperative evaluation of patients who are being considered for brain surgery. This information is critical in helping the neurosurgeon determine the best surgical approach and to minimize the risk of damaging critical brain structures. Additionally, EEG can be used to monitor the brain’s recovery after surgery, which can help predict the patient’s prognosis and inform the treatment plan. In recent years, the use of EEG has become increasingly sophisticated and has allowed for more precise and detailed monitoring of brain function during surgery. For example, high-resolution EEG techniques can be used to provide real-time information about the activity of specific brain regions. Additionally, developing wearable and portable devices in the future will allow continuous monitoring of brain function, providing real-time data on a patient’s condition.
In conclusion, EEG is a critical tool in the field of neurosurgery and has dramatically improved the ability of neurosurgeons to diagnose, treat, and monitor patients with neurological conditions. With continued advances in EEG technology, its use in neurosurgery will likely continue to grow and play an increasingly important role in improving patient outcomes.
Introduction
Analysis of EEG signals for neurosurgical patients is an important area of research that can improve the diagnosis and treatment of neurological disorders. Electro Encephalo Graphy (EEG) is a non-invasive technique that measures the brain’s electrical activity and provides valuable information about its function [1]. EEG signals have been used in neuroscience for decades, providing crucial insights into the underlying mechanisms of various neurological conditions, including epilepsy, brain tumors, and other neurodegenerative diseases [1,2].
EEG has provided the location and extent of brain tumors and other lesions such as epilepsy. In addition, it monitors patients intraoperatively to minimize risk to eloquent areas during brain surgery. Its use intraoperatively was first described by Penfield in 1939, where it was used to lateralize seizure origin in a patient with bitemporal epilepsy [3]. EEG also provides information on the effectiveness of new surgical techniques and medications used to treat neurological diseases. The analysis of EEG signals has traditionally relied on visual inspection and manual annotation by experienced neurophysiologists [4]. However, this approach is time-consuming, subjective, and prone to human error. Advances in computational methods and algorithms have enabled the development of automated EEG analysis tools that can provide more accurate and objective assessments of brain function [5]. These tools can potentially transform the field of neurosurgery by improving the speed, accuracy, and reliability of EEG-based diagnosis and treatment.
One of the key challenges in advancing analytics of EEG signals for neurosurgery patients is to develop algorithms that can accurately identify specific EEG patterns indicative of different neurological conditions [6]. For example, EEG signals are used to determine a brain tumor’s location and extent, seizure activity’s presence, or the effects of brain injury. These algorithms must be robust and reliable, capable of accurately detecting EEG patterns in the presence of noise and other confounding factors. Another critical challenge is to develop algorithms that can integrate EEG data with other imaging modalities, such as functional Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET), to provide a more comprehensive understanding of brain function [7,8]. This data integration from multiple sources will enable the development of more accurate and effective treatment plans for neurosurgery patients.
Analysis of EEG signals in neurosurgical patients is a rapidly growing field that can revolutionize the diagnosis and treatment of neurological disorders. In this literature review, we aim to highlight the role of EEG in treating neurosurgical conditions, its limitations in diagnosis and monitoring, and future objectives in the field.
Overview of EEG
German physicist Hans Berger first discovered EEG (Electroencephalography) in 1929 [9]. This discovery proved to be a significant breakthrough in diagnosing neurological and psychiatric conditions. EEG has the ability to measure the electrical activity of the brain by recording voltage differences between neurons. The collected EEG signals are amplified, digitalized, and sent to a computerized device for storage and data processing. Analyzing EEG data is an exceptional way of studying cognitive processes. EEG has been used to diagnose neurological conditions, monitor patients intraoperatively and postoperatively, and monitor treatment. Different types of EEG recording, such as standard/ routine EEG, sleep EEG, short-term video-EEG, long-term video EEG, continuous EEG, and invasive EEG, have been mentioned in the literature [10]. Invasive EEG monitoring with intracranial electrodes is indicated using either subdural electrodes (strips and grids) or depth electrodes (stereo-EEG) in selected patients undergoing presurgical evaluation when the epileptic focus to be resected cannot be localized with sufficient confidence using non-invasive methods.
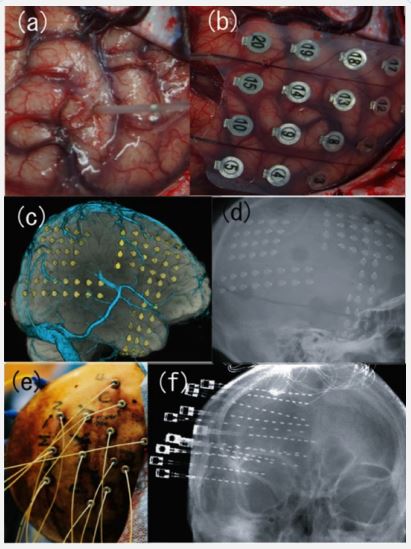
Intracranial electrodes encompass two types of electrodes, i.e., depth and subdural grids/strip electrodes (Figure 1a/1b) [11]. The techniques and types of recordings used by institutions differ. Most major epilepsy centers in Japan and North America use subdural electrodes or a combination of both electrodes (Figure 1c/1d). European epilepsy centers use stereotactically-inserted depth electrodes (stereoelectroencephalography, SEEG), introduced by Bancaud and Talairach in the 1950s (Figure 1e/1f) [12]. Additionally, the choice of electrodes depends on the areas that need to be explored. Invasive recordings with subdural electrodes are indicated for a precise description of seizure onset and spread on the cortical surface and provide a precise definition of the functional cortex and differecne in the interface between epileptogenic zones and functional cortex. However, subdural electrodes are limited because they cannot explore deeper generators, such as the insula and amygdala-hippocampal complex [11,13]. However, depth electrodes have the penetrating ability and are indicated when recording is needed from these deep brain structures. However, the main drawback of depth electrodes is limited spatial sampling and difficulties in precise anatomical delineation between contiguous cortical regions of the epileptogenic zone and functional cortex [11,13]. Therefore, carefully considering their advantages and disadvantages must be weighed to decide the electrode, technique, and location for electrode implantation with a hypothesis about the epileptogenic zone derived from a non-invasive evaluation.
Most signal power originates from rhythmic oscillations in a frequency bandwidth below 1 Hz to approximately 40 Hz. Also, higher frequencies can be measured up to 100 Hz [14]. This frequency range is subdivided into smaller, functional ranges with associated names [14]. The alpha rhythm includes medium-frequency activity (8–13 Hz) and usually indicates states of relaxed wakefulness in healthy adults [15]. The amplitude of these oscillations is typically substantial and can range up to several tens of mV. This wave type is also common during resting periods when people have their eyes closed, with the largest amplitudes in the occipital areas. Based on this finding, researchers have argued that alpha waves constitute a neural correlate of cognitive inactivity, also called cortical “idling” [16].
On the other hand, studies with evoked EEG activity (i.e., ERP investigations) have found that alpha rhythms may indicate different forms of information processing in which different alpha sub-bands (e.g., 8–10 and 10–13 Hz), which are dedicated to different functional processes [17,18]. The Alpha rhythms originating from sensorimotor areas are also known as µ rhythms and are further subdivided into lower and higher µ rhythms [19]. Large amplitudes constitute resting sensorimotor areas. Beta oscillations are characterized by medium to high-frequency activity (13–30 Hz). They are related to various mental states, such as active concentration, task engagement, excitement, anxiety, attention, or vigilance. They are also markers for sensorimotor activity. The amplitudes of this wave are usually in the mV— beta activity primarily includes an excitatory mechanism [20]. Gamma oscillations are characterized by very high-frequency activity (30–200 Hz, but typically not measurable by EEG when higher than 100 Hz). These oscillations are closely associated with arousal and perceptual binding mechanisms (i.e., integrating various aspects of a stimulus into a coherent overall perception). The amplitudes are relatively small, usually between 1 and 2 mV [21]. Delta waves are characterized by very lowfrequency activity (below 1–4 Hz), which usually relates to deep and unconscious sleep in healthy humans. Delta waves are also associated with pathologic neural states, such as coma or the loss of consciousness. Generally, delta activity diminishes with age, which indicates that delta activity is primarily an inhibitory mechanism [22]. Theta waves occur as low-frequency activity (4–8 Hz) and are characteristically associated with specific sleep states, drowsiness, and meditation. This type has been associated with mental effort, suggesting that attention is directed to an existing stimulus. In general, the amplitude of theta waves is typically between 8 and 10 mV [23].
Artificial Neural Networks (AAN) using raw EEG data have the ability to localize brain tumors [24]. The AAN used in the proposed system was a feed forward back propagation neural network. However, the EEG signals initially contain artifacts from both the subject and equipment interfaces that need to be filtered out [24]. In addition, the same systems can also be used for the identification of epileptogenic foci. Therefore, many papers have proposed using machine learning algorithms to automatically classify focal and non-focal EEG signals. This automatic classification as an analysis tool helps neurosurgeons identify focal areas for surgery while processing substantial data collected during several days of patient monitoring.
Figure 1: Various types of intracranial electrodes. Intraoperative view of (a) depth electrode and (b) subdural grid electrode. Threedimensional reconstructed computed tomography (c) and radiography of implanted subdural grid electrodes (d). Intraoperative view (e) and radiography of stereotactically inserted depth electrodes (f) (stereoelectroencephalography, SEEG).
EEG current treatment guidelines
EEG is used to diagnose neurological disorders such as epilepsy, stroke, and sleep disorders, monitor brain activity during surgery, and evaluate brain death. For example, in Neurosurgery, EEG is used in mapping the brain to identify eloquent areas preoperatively, monitoring brain function intraoperatively, evaluating the effects of anesthesia, determining the extent of brain injury, and monitoring brain activity after surgery.
EEG can potentially facilitate functional brain mapping with temporal resolution in the millisecond range. EEG recordings from electrodesare correlated with functional MRI to provide information on the location of the epileptogenic lesions. EEGcorrelated functional MRI (EEG-fMRI) measures changes in oxygenation in response to (interictal) epileptic events [25,26]. A study by Zijlmmans et al. showed that EEG-fMRI is useful in the localization of epileptogenic foci in complexcases [27]. It was also valuable in patients with presumed multifocality, as EEGfMRI could emphasize one of the foci. Finally, it showed EEGfMRI could tip the scales in favorof surgery in complex cases [27].
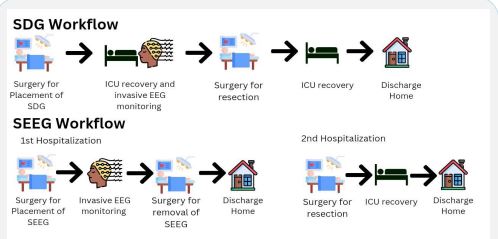
Using intracranial EEG for functional electrical stimulation mapping (ESM) is essential for patients with drug-resistant epilepsy. This is because intracranial EEG allows the ability to perform ESM by applying small currents to the same recording arrays and observing behavior responses. This allows the characterization of the functional anatomy of the area of interest by the principle of Bartholow that electrical brain stimulation elicits consistent and observable responses [28]. Resective epilepsy surgery is based on the use of invasive EEG monitoring in the form of subdural grids (SDG) or depth electrodes (stereo-electroencephalography, SEEG). SEEG and SDG involve the patient undergoing surgery to place the grid or electrode. The patient is then monitored to identify the lesion, following which the grid or electrode is removed, and the lesion is resected [29] (Figure 3). SEEG is currently less used than SDG due to its more invasive nature. However, the insertion of SEEG does not require a craniotomy and can be achieved using burr holes, and its removal of the electrodes is simple and does not require surgery [30]. A wake craniotomy is used in the mapping and resection of eloquent regions of the brain where imaging is not sufficiently sensitive.It has gained popularity due to better neurosurgical and postoperative outcomes. Invasive EEG in the form of Electrocorticography (ECoG) or Intracranial Electroencephalography (iEEG) is used to monitor after discharge during stimulation. Direct Cortical Electrical Stimulation (DCES), also called cortical stimulation mapping, is frequently performed with ECoG recording for functional mapping of the brainand identification of critical structures [31].
Figure 3: A pictorial description of EMS. SDG Workflow: Patients
undergo surgery for SDG implantation, after which they are monitored in the ICU. After monitoring is complete, the patient undergoes a subsequent surgery to remove the electrodes and resect
the epileptogenic focus. After surgery, the patient recovers in the
ICU and is discharged home. The whole process takes place in a
single hospitalization.
SEEG Workflow: Patients undergo two hospitalizations. In the first
hospitalization, SEEG electrodes are placed, and patients undergo
monitoring. After completion of monitoring, the patient undergoes surgery for SEEG electrode removal and is discharged home.
The patient is then admitted for a second time for resection of the
epileptogenic focus. After the surgery, the patient recovers in the
ICU and is discharged home.
Limitations in the use of EEG
Electroencephalography (EEG) is a widely used diagnostic tool in neurosurgery, but it does have some limitations. The two non-invasive diagnostic imaging techniques used in humans are metabolic-based, including fMRI (Functional MRI), PET (Positron Emission Tomography), near-infrared spectrometry, etc., and electrophysiological based, including EEG and magnetroelectrography [45]. A third technique, the interference technique consisting of transcranial magnetic imaging, will not be discussed here. Metabolic techniques classically have a good special resolution but a relatively poor temporal one, whereas electrophysiological techniques have an excellent temporal resolution but a poor spacial one [45]. The electrical activity recorded by the electrodes usually summates the excitatory and inhibitory postsynaptic potentials of neurons in the most superficial layers of the cortex [46]. The electrodes placed on the scalp or brain require the activation of quite a large area of a few centimeter squares to produce adequate potentials to be recorded [46].
Additionally, the propagation of electrical activity through physiological pathways or volume conduction through extracellular spaces can give misleading information on the location of the lesion [46]. Such a volume-conduction-induced mixture leads to confounding and poor spatial resolution of about 5 cm to 9 cm [47,48]. EEG measures electrical activity in the brain but is unable to provide detailed information about the exact location of the activity. This makes it difficult to determine the precise location of the source of brain activity and can limit the accuracy of EEG in guiding the identification of epileptic foci or neurosurgical procedures. EEG provides information about the brain’s electrical activity over time but does not provide a continuous, real-time record of brain activity [49,50]. This is known as temporal resolution. Measurement of electrical activity on the surface of the scalp is affected by various factors such as the thickness of the skull and brain tissue conductivity leading. This can make it difficult to identify very short-lived or rapidly changing events in the brain. Although theoretically, the temporal resolution of EEGs is excellent, its actual one is lowered due to the same physical phenomenon lowering its spatial resolution. However, improving the latter mechanically improves temporal resolution [48]. EEG recordings can be affected by movements and muscle activity interference, which can lead to artifacts in the EEG signal that make it difficult to interpret the data accurately [51]. The accuracy of EEG depends on the placement of electrodes on the scalp, and minor variations in placement can significantly impact the EEG signal [52,53]. This can make it difficult to obtain consistent and reliable EEG recordings. Additionally, EEG has a limited ability to evaluate deep brain structures. The skull and brain tissue’s electrical activity gets dampened, making it difficult to obtain accurate information from the deeper structures. This can limit the ability of EEG to provide complete information about the brain and to guide specific neurosurgical procedures.
Intracranial EEG monitoring is an excellent method for studying epileptogenic human brain anatomy. Unfortunately, introducing intracranial electrodes poses risks to the patient [54,55]. Its risks and limitations include a high cost, patient discomfort, aseptic meningitis, patient immobility, limited time for assessment, the need for staged neurosurgical procedures, and inherent limitations in localizing the lesion [54-58]. Patient discomfort can be due to extensive scalp exposure and muscle dissection. Incidence of hemorrhage in recent case series ranges from 0-12%, concluding life-threatening hemorrhage can and does occur, albeit at a low rate [59]. Additionally, wires attached to the electrodes are cumbersome and, if caught when the patient moves, place painful traction to the dura.
Despite these limitations, EEG remains a valuable tool in neurosurgery and is used in combination with other diagnostic tools, such as Magnetic Resonance Imaging (MRI) and Computed Tomography (CT), to provide a complete picture of brain function.
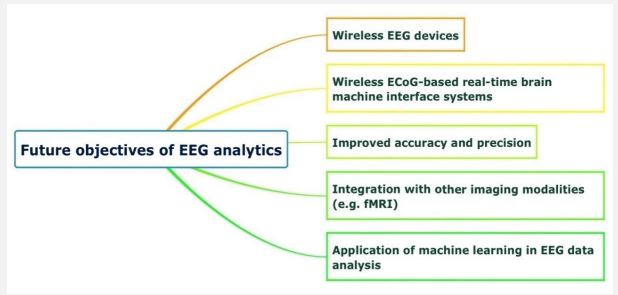
Future objectives in the use of EEG in neurosurgery
Many wireless EEG devices are currently available in the market and offer many improvements from previous generations, from more advanced electrode designs to enhanced signal quality and amore comprehensive range of configurations for different use cases [60]. For example, the emergence of dry electrodes overcomes the limitation of gel-based electrodes in the recording duration as they dry out with time and cannot produce long-term recordings. However, the trade-off with dry electrodes has been their signal quality, and much research is being conducted on minimizing noise using alternative shapes and materials of dry electrodes [61]. In addition, hardware miniaturization and the creation of wearable EEG that allows for signal recording outside the lab as people move about has also been an area of interest [62]. Other research avenues include the number and placement of EEG and reference channels, additional external and internal sensors, minimization of external noise, higher sampling rates to record high-frequency brain activity, improved digital resolution, and longer battery life [60].
Wireless implantable Brain-Machine Interfaces (BMIs) are a promising tool to monitor intracranial EEG and provide stimulation for patients with motor disabilities [63,64]. For example, a novel BMI wireless device customized by Yan et al. using thirtytwo subdural electrodes was evaluated in two awake macaque monkeys. It was shown to be successful in recording Electro-corticography (ECoG) signals [65]. In another project, a Wireless Human Ecog-Based Real-Time BMI System (W-HERBS) was developed by Matsushita et al. using high-density subdural electrodes recording 128-channel ECoG signals at a sampling rate of 1 kHz [66]. These results encourage further studies on ECoG-based BMI systems and their potential application in the clinical setting.
With advances in EEG technology, improving the accuracy and precision of EEG recordings is an important goal in the field. New quality assurance processes are being established to help identify and quantify factors affecting the accuracy of SEEG implantation [67,68]. Furthermore, to provide a more comprehensive picture of brain activity, there has been a growing interest in integrating EEG recordings with data from other imaging modalities [69]. Simultaneous EEG-fMRI recordings offer various advantages: It allows the detection of cortical areas involved in interictal and ictal epileptic activity, improve accuracy in detecting neural activity since data from fMRI alone can be influenced by hemodynamic alterations secondary to pathological conditions or drugs, as well as enabling the evaluation of functional connectivity in unconscious patients [70-72].
The application of Machine Learning (ML) in EEG data analysis is an area that has received significant attention in the last two decades. EEG signal-based Brain-Computer Interfaces (BCI) have been developed to enable patients with voice impairment to communicate with their surroundings [73,74]. However, current ML models require word or sentence prompts, and models for continuous sentence prediction, which would enable communication in people with cognitive disabilities, is still an area of ongoing research [74,75]. ML models have also demonstrated excellent performance in predicting seizure onset based on patterns in pre-ictal EEG signals, as well as seizure classification and localization of the seizure onset zone, which is the most important predictor of positive surgical outcome. ML is also increasingly utilized in the study of cognitive performance. The potential for ML to identify neural activity patterns associated with specific tasks provides an avenue for developing improved neuromodulatory systems [76]. ML-powered outcomes prediction models using EEG signals are also a growing field of interest, with studies on various disease conditions such as stroke and dementia [77,78].
An ML model analyzing EEG data to classify performance during simulated surgery was developed by Natheir et al. with the aim of enhancing neurosurgical training [60]. In this project involving twenty-one participants, a ML model was trained to differentiate between skilled and less-skilled performance on brain tumor resection simulations on the Neuro VR™ platform using data from EEG recorded during the procedures. Markers of expertise that were identified include lower TBR and significantly higher low-alpha (8–10 Hz), beta (13–30 Hz), beta 1 (15–18 Hz), and beta 2 (19–22 Hz) frequency bands. Such systems can enhance neurosurgical education by enabling more quantitative, formative, and summative assessment of surgical performance [60].
Conclusion
EEG is a test used to evaluate the electrical activity of the brain. It has been widely used to diagnose and monitor various neurological conditions. In neurosurgery, EEG has been used for mapping brain areas, detecting epileptogenic foci, intraoperatively monitoring brain function, and in the form of invasive EEG to treat epilepsy and other neurological illnesses. However, there are still some limitations to its use. EEG cannot provide a sufficient spatial resolution that affects its temporal resolution. Invasive EEG has complications such as hemorrhage and infection. The implantable electrodes must be removed surgically, and external wires are cumbersome. Future research should be directed toward developing machine learning algorithms for automatically interpreting EEG recordings and improving patient usability.
References
- Jameson LC, Janik DJ, Sloan TB. Electrophysiologic monitoring in neurosurgery. Anesthesiology clinics. 2007; 25: 605-630.
- Sramka M, Brozek G, Bures J, et al. Functional ablation by spreading depression: Possible use in human stereotactic neurosurgery. Applied neurophysiology. 1977; 40: 48-61
- Penfield W. The Epilepsies: With a note on Radical Therapy. The New England Journal of Medicine 1939; 221.
- Vaid S, Singh P, Kaur C, editors. EEG signal analysis for BCI interface: A review. 2015 fifth international conference on advanced computing & communication technologies; 2015: IEEE.
- Acharya UR, Sree SV, Swapna G, et al. Automated EEG analysis of epilepsy: A review. Knowledge-Based Systems. 2013; 45: 147-165.
- Raghu S, Sriraam N. Classification of focal and non-focal EEG signals using neighborhood component analysis and machine learning algorithms. Expert Systems with Applications. 2018; 113: 18-32.
- Liu Z, Ding L, He B. Integration of EEG/MEG with MRI and fMRI. IEEE engineering in medicine and biology magazine. 2006; 25: 46-53.
- Neuner I, Rajkumar R, Brambilla CR, et al. Simultaneous PETMR-EEG: technology, challenges and application in clinical neuroscience. IEEE transactions on radiation and plasma medical sciences. 2018; 3: 377-385.
- Tudor M, Tudor L, Tudor KI. [Hans Berger (1873-1941)-the history of electroencephalography]. Acta Med Croatica. 2005; 59: 307-313.
- Beniczky S, Schomer DL. Electroencephalography: Basic biophysical and technological aspects important for clinical applications. Epileptic Disord. 2020; 22: 697-715.
- Zumsteg D, Wieser HG. Presurgical evaluation: Current role of invasive EEG. Epilepsia. 2000; 41: S55-S60.
- Bancaud J, Angelergues R, Bernouilli C, et al. Functional Stereotaxic Exploration (SEEG) of epilepsy. Electroencephalogr Clin Neurophysiol. 1970; 28: 85-86.
- Enatsu R, Bulacio J, Najm IM, et al. Combining stereo-electroencephalography and subdural electrodes in the diagnosis and treatment of medically intractable epilepsy. Journal of Clinical Neuroscience. 2014; 21: 1441-1445.
- Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Oxford University Press; 2017. (Schomer DL, Lopes da Silva FH, editors.).
- Berger H. Über das Elektrenkephalogramm des Menschen. Archiv für Psychiatrie und Nervenkrankheiten. 1929; 87: 527-570.
- Pfurtscheller G, Stancák A, Neuper C. Event-Related Synchronization (ERS) in the alpha band--an electrophysiological correlate of cortical idling: a review. International journal of psychophysiology: Official journal of the International Organization of Psychophysiology. 1996; 24 1-2: 39-46.
- Niedermeyer E. Alpha rhythms as physiological and abnormal phenomena. Int J Psychophysiol. 1997; 26: 31-49.
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Brain Res Rev. 1999; 29: 169-195.
- Pfurtscheller G, Neuper C, Krausz G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin Neurophysiol. 2000; 111: 1873-1879.
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999; 110: 1842-1857.
- Hughes JR. Gamma, fast, and ultrafast waves of the brain: Their relationships with epilepsy and behavior. Epilepsy Behav. 2008; 13: 25-31.
- Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: Neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002; 3: 679-693.
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006; 132: 180-211.
- Murugesan M, Sukanesh R, editors. Automated Detection of Brain Tumor in EEG Signals Using Artificial Neural Networks. 2009 International Conference on Advances in Computing, Control, and Telecommunication Technologies; 2009; 28-29.
- Krakow K, Woermann F, Symms M, et al. EEG-triggered functional MRI of interictal epileptiform activity in patients with partial seizures. Brain. 1999; 122: 1679-1688.
- Bénar C-G, Gross DW, Wang Y, et al. The BOLD response to interictal epileptiform discharges. Neuroimage. 2002; 17: 1182-1192.
- Zijlmans M, Huiskamp G, Hersevoort M, et al. EEG-fMRI in the preoperative work-up for epilepsy surgery. Brain. 2007; 130: 2343-2353.
- Bartholow R. Experiments on the functions of the human brain. British medical journal. 1874; 1: 727.
- Salehi A, Yang PH, Smyth MD. Single-center cost comparison analysis of stereoelectroencephalography with subdural grid and strip implantation. Journal of Neurosurgery: Pediatrics. 2022; 29: 568-574.
- Taussig D, Montavont A, Isnard J. Invasive EEG explorations. Neurophysiologie Clinique/Clinical Neurophysiology. 2015; 45: 113-119.
- Shah AK, Mittal S. Invasive electroencephalography monitoring: Indications and presurgical planning. Ann Indian Acad Neurol. 2014; 17: S89-94.
- Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006; 4: 103-112.
- Kreiter KT, Copeland D, Bernardini GL, et al. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. 2002; 33: 200-208.
- Bergamasco B, Bergamini L, Doriguzzi T, et al. EEG sleep patterns as a prognostic criterion in post-traumatic coma. Electroencephalogr Clin Neurophysiol. 1968; 24: 374-377.
- Claassen J, Mayer SA, Hirsch LJ. Continuous EEG monitoring in patients with subarachnoid hemorrhage. J Clin Neurophysiol. 2005; 22: 92-98.
- Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004; 115: 2699-2710.
- Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997; 103: 607-615.
- Hilz MJ, Litscher G, Weis M, et al. Continuous multivariable monitoring in neurological intensive care patients-Preliminary reports on four cases. Intensive Care Med. 1991; 17: 87-93.
- Fried I, Wilson CL, Maidment NT, et al. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients. Technical note. J Neurosurg. 1999; 91: 697-705.
- Sharma R, Arora C, Rehalia A, et al. Fruitfly optimizer with deep neural network for the detection of brain tumours using EEG signals. Journal of Information and Optimization Sciences. 2022 2022/01/02; 43: 63-70.
- De Jesus O, Fogwe DT, Mesfin FB, et al. Neuromodulation Surgery For Psychiatric Disorders. StatPearls Publishing, Treasure Island (FL); 2022 2022. eng.
- Holtzheimer PE, Mayberg HS. Deep brain stimulation for psychiatric disorders. Annu Rev Neurosci. 2011; 34: 289-307.
- Valencia-Alfonso CE, Luigjes J, Smolders R, et al. Effective deep brain stimulation in heroin addiction: A case report with complementary intracranial electroencephalogram. Biol Psychiatry. 2012;71: e35-e37.
- Mithani K, Meng Y, Abrahao A, et al. Electroencephalography in Psychiatric Surgery: Past Use and Future Directions. Stereotactic and Functional Neurosurgery. 2019; 97: 141-152.
- Burle B, Spieser L, Roger C, et al. Spatial and temporal resolutions of EEG: Is it really black and white? A scalp current density view. International Journal of Psychophysiology. 2015; 97: 210- 220.
- Smith SJM. EEG in the diagnosis, classification, and management of patients with epilepsy. Journal of Neurology, Neurosurgery & amp; Psychiatry. 2005; 76: ii2-ii7.
- Nunez PL, Silberstein RB, Cadusch PJ, et al. A theoretical and experimental study of high resolution EEG based on surface Laplacians and cortical imaging. Electroencephalography and clinical neurophysiology. 1994; 90: 40-57.
- Babiloni F, Cincotti F, Carducci F, et al. Spatial enhancement of EEG data by surface Laplacian estimation: the use of magnetic resonance imaging-based head models. Clinical Neurophysiology. 2001; 112: 724-727.
- Krohn S, von Schwanenflug N, Waschke L, et al. A spatiotemporal complexity architecture of human brain activity. Science Advances. 2023; 9: eabq3851.
- Zanos S. Neural Correlates of High-Frequency Intracortical and Epicortical Field Potentials. The Journal of Neuroscience. 2009; 29: 3673-3675.
- Anderer P, Roberts S, Schlögl A, et al. Artifact processing in computerized analysis of sleep EEG–a review. Neuropsychobiology. 1999; 40: 150-157.
- Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clinical Neurophysiology. 2001; 112: 713-719.
- Ryynanen O, Hyttinen JA, Laarne PH, et al. Effect of electrode density and measurement noise on the spatial resolution of cortical potential distribution. IEEE transactions on biomedical engineering. 2004; 51: 1547-1554.
- Fountas K, Smith J. Subdural electrode-associated complications: A 20-year experience. Stereotact Funct Neurosurg. 2007; 85: 264-272.
- Hamer H, Morris H, Mascha E, et al. Complications of invasive video-EEG monitoring with subdural grid electrodes. Neurology. 2002; 58: 97-103.
- Johnston J, Mangano F, Ojemann J, et al. Complications of invasive subdural electrode monitoring at St. Louis Children’s Hospital, 1994–2005. J Neurosurg. 2006; 105: 343-347.
- Lee W, Lee J, Lee S, et al. Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol. 2000; 54: 346-351.
- Musleh W, Yassari R, Hecox K, et al. Low incidence of subdural grid-related complications in prolonged pediatric EEG monitoring. Pediatr Neurosurg. 2006; 42: 284-287.
- Blount JP, Cormier J, Kim H, et al. Advances in intracranial monitoring. Neurosurgical Focus FOC. 2008 01 Sep 01. 2008; 25: E18.
- Natheir S, Christie S, Yilmaz R, et al. Utilizing artificial intelligence and electroencephalography to assess expertise on a simulated neurosurgical task. Comput Biol Med. 2023; 152: 106286.
- Kam JWY, Griffin S, Shen A, et al. Systematic comparison between a wireless EEG system with dry electrodes and a wired EEG system with wet electrodes. Neuroimage. 2019; 184: 119- 129.
- Casson AJ. Wearable EEG and beyond. Biomed Eng Lett. 2019; 9: 53-71.
- Su Y, Routhu S, Moon KS, et al. A Wireless 32-Channel Implantable Bidirectional Brain Machine Interface. Sensors (Basel). 2016; 16.
- Choi JR, Kim SM, Ryu RH, et al. Implantable Neural Probes for Brain-Machine Interfaces - Current Developments and Future Prospects. Exp Neurobiol. 2018; 27: 453-471.
- Yan T, Suzuki K, Kameda S, et al. Intracranial EEG Recordings of High-frequency Activity from a Wireless Implantable BMI Device in Awake Nonhuman Primates. IEEE Trans Biomed Eng. 2022;PP.
- Matsushita K, Hirata M, Suzuki T, et al. A Fully Implantable Wireless ECoG 128-Channel Recording Device for Human Brain-Machine Interfaces: W-HERBS. Front Neurosci. 2018; 12: 511.
- Rodionov R, O’Keeffe A, Nowell M, et al. Increasing the accuracy of 3D EEG implantations. J Neurosurg. 2019: 1-8.
- Lu C, Chen S, An Y, et al. How can the accuracy of SEEG be increased?-an analysis of the accuracy of multilobe-spanning SEEG electrodes based on a frameless stereotactic robot-assisted system. Ann Palliat Med. 2021; 10: 3699-3705.
- Jorge J, van der Zwaag W, Figueiredo P. EEG-fMRI integration for the study of human brain function. Neuroimage. 2014 ; 102: 24- 34.
- Vitali P, Di Perri C, Vaudano AE, et al. Integration of multimodal neuroimaging methods: a rationale for clinical applications of simultaneous EEG-fMRI. Funct Neurol. 2015; 30: 9-20.
- Warbrick T. Simultaneous EEG-fMRI: What Have We Learned and What Does the Future Hold? Sensors (Basel). 2022; 22.
- Mele G, Cavaliere C, Alfano V, et al. Simultaneous EEG-fMRI for Functional Neurological Assessment [Review]. Frontiers in Neurology. 2019 2019; 10.
- Värbu K, Muhammad N, Muhammad Y. Past, Present, and Future of EEG-Based BCI Applications. Sensors. 2022; 22: 3331.
- Lazarou I, Nikolopoulos S, Petrantonakis PC, et al. EEG-Based Brain-Computer Interfaces for Communication and Rehabilitation of People with Motor Impairment: A Novel Approach of the 21 (st) Century. Front Hum Neurosci. 2018; 12: 14.
- Shah U, Alzubaidi M, Mohsen F, et al. The Role of Artificial Intelligence in Decoding Speech from EEG Signals: A Scoping Review. Sensors (Basel). 2022; 22.
- Mirchi N, Warsi NM, Zhang F, et al. Decoding Intracranial EEG With Machine Learning: A Systematic Review. Front Hum Neurosci. 2022; 16: 913777.
- Islam MS, Hussain I, Rahman MM, et al. Explainable Artificial Intelligence Model for Stroke Prediction Using EEG Signal. Sensors (Basel). 2022; 22.
- Rutkowski TM, Abe MS, Otake-Matsuura M. Neurotechnology and AI Approach for Early Dementia Onset Biomarker from EEG in Emotional Stimulus Evaluation Task. Annu Int Conf IEEE Eng Med Biol Soc. 2021; 2021: 6675-6678.