Research article
Volume 2, Issue 3
Current Treatment of Depression in Children with Autism and Intellectual and Developmental Disabilities
J Nikki Steinsiek1*; Aaron L Fox2 ; Jessica LC Blanks2 ; Gabrielle E Hodgins3 ; Juliana W Stone2 ; James E Bedford3
1Children’s National Hospital, Washington, DC, US.
2University of North Carolina School of Medicine, Chapel Hill, NC, US.
3Department of Psychiatry, University of North Carolina School of Medicine, Chapel Hill, NC, US.
*Corresponding Author:
J Nikki Steinsiek
Tel: 202-476-4000
Email: nsteinsiek@gmail.com
Received : Feb 03, 2023 Accepted : Mar 24, 2023 Published : Mar 31, 2023 Archived : www.meddiscoveries.org
Citation: Steinsiek JN, Fox AL, Blanks JLC, Hodgins GE, Stone JW, et al. Current Treatment of Depression in Children with Autism and Intellectual and Developmental Disabilities. Med Discoveries. 2023; 2(3): 1029.
Copyright: © 2023 Steinsiek JN. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
There are no evidence-based guidelines for the treatment of depression in Autism Spectrum Disorder (ASD). We describe prescribing practices for youth with depression and ASD and/or Intellectual and Developmental Disabilities (IDDs). Data was queried for youth with ASD and/or IDDs ages 10-18 with diagnoses of depression seen in an academic outpatient psychiatry clinic between 2015 and 2020.
The final sample included 54 youth with ASD, 18 with ASD plus comorbid IDDs, and 14 with IDDs. The most common medications prescribed were fluoxetine and sertraline. Of those on medication for depression, 30% required augmentation. Among youth who were prescribed an antidepressant and required a second psychopharmacologic agent for augmentation, 17% were prescribed an antipsychotic drug. Polypharmacy was more common in those with ASD plus IDDs and psychotherapy was less commonly used in those with IDDs.
Keywords: Autism spectrum disorder; Intellectual disability; Psychopharmacology; Child and adolescent psychiatry.
Introduction
Major Depressive Disorder (MDD) is a disabling condition affecting 10.6% (7.6–14.6, 95% Confidence Interval (CI)) of youth under the age of 18 with Autism Spectrum Disorder (ASD) [1]. The reported rates of depressive disorders in youth with ASD are comparable, if not higher, than that of the general neurotypical population [1-3]. The elevated rate of depressive disorders in persons with ASD continues into adulthood, with numerous studies finding the prevalence of depression to far exceed that of the general population [4,5]. Unfortunately, despite evidence to suggest a dire need for better management and treatment of mood disorders in youth with ASD, depression in ASD remains understudied and underdiagnosed [6].
There are currently no evidence-based guidelines for the treatment of depression in children and adolescents with ASD. Although multiple clinical trials have investigated the treatment of irritability, hyperactivity, and insomnia in children and adolescents with ASD this is not the case for depression treatment [7]. While selective serotonin reuptake inhibitors (SSRIs) are one of the most commonly prescribed classes of medications for youth with ASD [8], there are no reported randomized, double-blind, placebo-controlled trials of pharmacological agents for depression in ASD in either adults or children. Empirical evidence supports the use of SSRIs for depression in neurotypical youth, however, evidence for their efficacy in youth with ASD is lacking [9]. Previous clinical trials of SSRIs, for autism features, obsessive compulsive disorders, anxiety, depression, and aggression,suggest that SSRIs may be less well tolerated in youth with ASD, with a higher frequency of behavioral side effects (e.g., irritability, insomnia) than reported in their neurotypical peers [10,11].
To our knowledge, there are no studies that describe current prescribing practices specifically for the treatment of depression in youth with ASD. The aim of this single-site, clinic-based naturalistic study is to fill this gap in the existing literature, providing pilot data to be used in the design of future pharmacologic trials for the management of MDD in youth with ASD. Studying the safety and efficacy of psychopharmacologic treatments for this vulnerable population is of vital importance, as we currently lack evidence to inform optimal care. For example, in trials of stimulants, medication was found to be significantly less effective and significantly more likely to cause side effects including aggression and irritability [12]. If certain medications for depression are less well tolerated, it is crucial to determine so to avoid disparate harm or lack of benefit. Or, if certain medications are more likely to be continued, this could indicate relatively good efficacy or tolerability. To this end, our study describes current prescribing practices for children with depression and ASD and also compares prescribing practices between youth with ASD and those with IDDs to assess if the described practices are unique to those with ASD or generally applicable to the broader neurodiverse community. As there are many behavioral and neurobiological features of ASD that may uniquely contribute to depression presentations, we hypothesized that there would be differences in the type and number of medications prescribed for depression. In addition, we hypothesized that there would be differences in psychiatric symptoms and comorbidities that influence prescribing practices.
Methods
Participants
The present study was a medical chart review conducted in the Department of Psychiatry at a large suburban teaching hospital offering a full range of child, adolescent, and adult psychiatric services. The study population consisted of psychiatry outpatient’s ages 10-18 years old, who had a diagnosis of ASD, IDD, or both ASD and IDD, met inclusion criteria, and accessed psychiatric outpatient services from January 1, 2015 to January 1, 2020. Inclusion criteria included: youth ages 10-18; diagnosis of ASD or IDD; and diagnosis of a Depressive Disorder Not Otherwise Specified (DDNOS) or Major Depressive Disorder (MDD). In this paper the term depression will refer to both DDNOS and MDD. Exclusion criteria included: any present or former diagnosis of a substance use disorder, epilepsy, or history of pregnancy. Once prevalence of a depressive disorder was estimated, data analyses were restricted to those with a diagnosis of depression. Information was extracted from the most recent psychiatric evaluation by a data analysis specialist. Additional chart review was completed by two investigators to ensure integrity of extracted data and collect additional variables of interest.
Demographic variables
The following variables were extracted by the data specialist: gender, race, ethnicity, and age at last encounter in timeframe. The following were collected via chart review by investigators: gender, maternal level of education, history of trauma (including physical and emotional neglect as well as physical, emotional, and sexual abuse), history of child protective services (CPS) involvement, family history of depression, and history of preterm birth.
Diagnoses and treatment
Specified diagnoses were extracted from the problem list using ICD-9 and 10 codes. The most inclusive ICD codes for depressive disorders, anxiety disorders, other mood disorders, ASDs, and disability disorders were used. Extracted diagnoses were verified by chart review. Diagnosis of ADHD was determined by chart review as it was not included in the initial data query. Psychotropic medication names, classes, categories, doses, and frequencies at the most recent encounter were extracted by the data specialist. Extracted medication data were reviewed and edited during chart review to accurately reflect medications being prescribed to the patient at their most recent visit. Indications for each psychotropic medication, number of prior medication trials for depression, and regularity of medication use were extrapolated from documentation in clinic notes. Similar data were collected for supplements including vitamin D, zinc, folic acid, and omega-3 fatty acids. Additional variables collected during chart review included several non-pharmacologic therapies: eligibility for Individualized Education Plan (IEP) or 504 plan; receipt of intensive in-home therapy; engagement in psychotherapy; cognitive behavioral therapy; occupational therapy; physical therapy; speech therapy; and other specialized therapies.
Psychiatric symptoms
Psychiatric symptoms related to depression were extracted through chart review, primarily by searching for terms in psychiatric progress notes. Symptoms included depressed mood, guilt, poor concentration, psychomotor changes, appetite changes, sleep changes, irritability, self-injurious behaviors, suicidal ideation, psychotic features, verbal aggression, physical aggression, and catatonia. Maximum PHQ-9 scores were extracted by the data specialist.
Variable creation
Multinominal categorical variables were created for age, race, ethnicity, depression medication type, antidepressant type, and polypharmacy. Binary categorical variables were created for history of trauma, race, depression augmentation, antipsychotic-specific augmentation, and regularity of medication use. Continuous variables were generated for number of psychotropic medications currently being prescribed, number of medications indicated for depression, and number of nonpharmacologic services received. Depression medication type categories were defined as: no medication; antidepressant; mood stabilizer; antipsychotic; and two or more medications. Antidepressant medication categories were defined as: SSRI; SNRI or atypical antidepressant; and two or more antidepressants. Depression augmentation was defined as being on any two or more medications indicated for depression per chart notes. Antipsychotic augmentation for depression was defined as being on both an antipsychotic and an antidepressant with the antipsychotic having an indication for depression per chart notes.
Statistical analyses
First, all three groups (i.e., ASD, IDD, and ASD/IDD) were compared using a chi-square test, and Fisher’s exact test was used when >20% of the cell counts were five or less. A p-value of 0.05 was used to determine significance of all tests, apart from pairwise comparisons for which a p-value of 0.016 was used to account for multiple comparisons. Kruskal–Wallis tests were used to compare continuous variables across groups given non-normal distributions. Multinominal, ordinal, and logistical variables were considered; however given limitations of small cell counts were not performed.
Results
Cohort characteristics
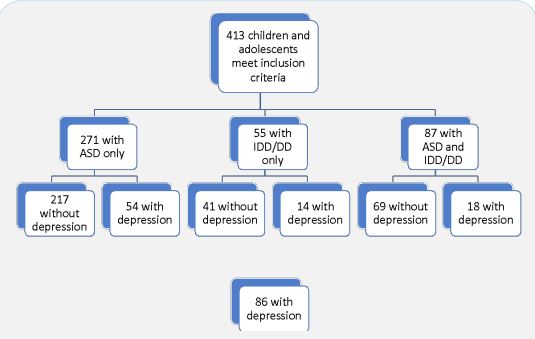
Data extraction revealed 413 youth diagnosed with ASD or IDD, without history of epilepsy or pregnancy that were seen at psychiatry outpatient clinics between January 2015 and January 2020. Of those, 86 had a documented diagnosis of depression. The three groups were closely similar in observed prevalence of depression: 21% (ASD), 24% (IDD); 22% (ASD and IDD (ASD/ IDD)). The final sample included 86 youth (45% girls, 63% white, and 17% black) with a median age of 16 (IQR: 13, 17). Figure 1 for the cohort selection flow diagram. Age, race, and ethnicity were closely similar between groups as shown in Table 1. There were trends towards differences in gender and prevalence of psychiatric comorbidities between groups. Among youth with IDD, 64% were female as compared to 39% identifying as female in both ASD and ASD/IDD groups (P = 0.243, Fisher’s Exact Test (FET)). Three youth reported gender uncertainty, one in each group. Anxiety prevalence was lower among youth with ASD (56%) as compared to 78% and 71% in the ASD/IDD and IDD groups respectively (P = 0.202, FET) and ADHD prevalence was lower among youth with ASD/IDD (39%) compared to 61% and 64% in the ASD and IDD groups respectively (P = 0.243, FET).
Table 1: Cohort Characteristics
| Cohort characteristics, n (%) | ||||||
|---|---|---|---|---|---|---|
| ASD Only | ASD + IDD | IDD | Total | P-value | ||
| Age | ||||||
| 10 -12 years | 9 (17) | 1 (5) | 3 (21) | 13 (15) | 0.739 | |
| 13 - 15 years | 15 (28) | 5 (28) | 4 (29) | 24 (28) | ||
| 16 - 18 years | 30 (55) | 12 (67) | 7 (50) | 49 (57) | ||
| Gender | ||||||
| Female | 21 (39) | 7 (39) | 9 (64) | 37 (43) | 0.243* | |
| Male | 33 (61) | 11 (61) | 5 (36) | 49 (57) | ||
| Gender Uncertainty | 1 | 1 | 1 | 3 | ||
| Race | ||||||
| White | 36 (67) | 10 (56) | 8 (57) | 54 (63) | 0.780* | |
| Black | 7 (13) | 4 (22) | 4 (29) | 15 (17) | ||
| Asian | 4 (7) | 1 (6) | 1 (7) | 6 (7) | ||
| Other | 2 (4) | 2 (11) | 0 | 4 (5) | ||
| Unknown/Refused | 5 (9) | 1 (6) | 1 (7) | 7 (8) | ||
| Ethnicity | ||||||
| Non-Hispanic | 44 (82) | 15 (83) | 13 (93) | 72 (84) | 0.750* | |
| Hispanic | 3 (6) | 2 (11) | 0 | 5 (6) | ||
| Refused/Unknown | 7 (13) | 1 (6) | 1 (7) | 9 (10) | ||
| Anxiety | ||||||
| Yes | 30 (56) | 14 (78) | 10 (71) | 54 (63) | 0.202* | |
| No | 24 (44) | 4 (22) | 4 (29) | 32 (37) | ||
| ADHD | ||||||
| Yes | 33 (61) | 7 (39) | 9 (64) | 49 (57) | 0.243* | |
| No | 21 (39) | 11 (61) | 5 (36) | 37 (43) | ||
Note: Total cohort, n = 86. ASD, autism spectrum disorder. IDD, intellectual and developmental disabilities. ADHD, attention deficit hyperactivity disorder. *Fischer’s exact test used when >20% cell counts < 5.
Adverse exposure history
The three groups were similar in prevalence of pre-term birth and family history of depression. Youth with ASD/IDD had the highest prevalence of CPS involvement at 23%, significantly more than youth with ASD only after pairwise comparisons (P = 0.008, FET; pairwise X2 = 8.5333, d.f. = 2, P = 0.003). Forty-three percent of youth with IDD had a history of trauma, as compared to 24% in those with ASD and 17% in those with ASD/IDD. The test of the hypothesis of no differences among the three subpopulations was inconclusive (P = 0.127, FET).
Psychiatric symptoms
Prevalence of suicidal ideation, depressed mood, poor concentration, sleep changes, self-injurious behavior, aggression, and catatonia were closely similar between groups. Youth with ASD/IDD had more psychomotor agitation than both youth with ASD (pairwise X2 = 7.7407, d.f. = 1, P = 0.005) and IDD (pairwise X2 = 6.6422, d.f. = 1, P = 0.010). Other notable differences, with inconclusive null hypothesis tests, included less expression of guilt in youth with ASD/IDD (11%; P = 0.124, FET), more appetite changes in youth with IDD (64%; P = 0.065; FET), more irritability in youth with IDD (86%; P = 0.130, FET), and more psychotic features in youth with ASD/IDD (28%; P = 0.261, FET).
Medications for depression
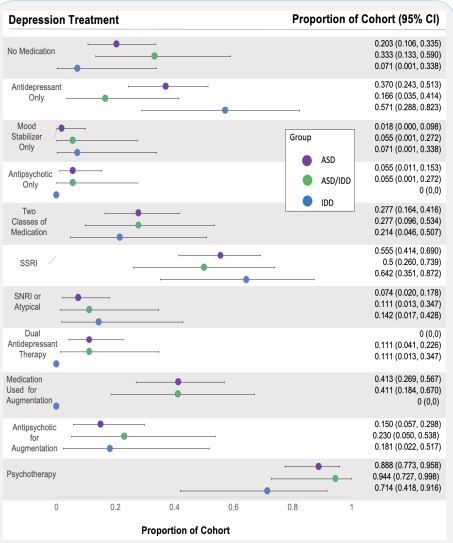
Of the 86 youth, 76 were prescribed one or more medications for depression, four were prescribed no medication for depression and six were prescribed no medication for any indication. Regular medication use was documented in 74% of the patient’s charts. Half of those on no medication were seen for evaluation through an affiliated community-based services program rather than a clinic for medication management. Of those on no medication for depression, all were prescribed stimulants for ADHD, one was prescribed an anxiolytic only, and one had three prior medication trials for depression all complicated by negative side effects. The most commonly prescribed medications for depression included fluoxetine (n=19, median dose 30 mg daily), sertraline (n=19, median dose 100 mg daily), escitalopram (n=14, median dose 20 mg daily), aripiprazole (n=12, median dose 5.75 mg daily), and lithium (n=10, median dose 600 mg daily). Select prescribing practices are presented in Figure 2. There were no identifiable between-group differences with regards to specific medications. To treat depression, 36% were prescribed an antidepressant only, 3% a mood stabilizer only, 14% an antipsychotic only, and 35% two or more types of medications for depression. Type of medication used for depression was similar between groups (P = 0.081, FET). Of those on SSRIs, SNRIs, and atypical antidepressants (n=64), 75% were on an SSRI alone, 12.5% were on an SNRI or atypical antidepressants, and 12.5% were on two of these medications. Type of antidepressant prescribed was closely similar between groups (P = 0.703, FET).
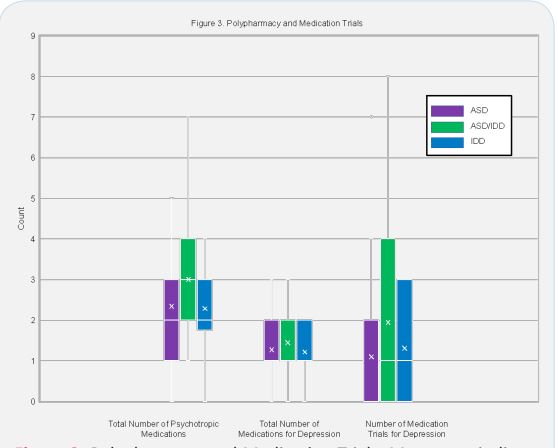
Polypharmacy
Figure 3 presents between group comparisons of total number of psychotropic medications, total number of medications for depression, and total number of depression medication trials. Of those on medication for depression, 30% were receiving a second medication for augmentation (total n = 76). Of those on antidepressants, 17% were on an antipsychotic for augmentation (total n = 64). Sixty-one percent of youth with ASD/IDD were prescribed antipsychotics, significantly more than those with ASD (pairwise X2 = 8.33, P = 0.004). Overall, 31% of subjects were prescribed an antipsychotic and 41% were prescribed three or more psychotropic medications for any indication. Of the antipsychotics used, all were atypical antipsychotics apart from one prescription for chlorpromazine which was indicated for psychosis rather than DDNOS. Antidepressant augmentation and number of medications for depression were similar between groups. Polypharmacy was more common in those with ASD/IDD however null hypothesis testing was inconclusive (Kruskal-Wallis, P = 0.418).
Non-pharmacologic treatments and supportive therapies
The median number of non-pharmacologic therapies and supports being utilized were similar between groups. On average, youth were receiving four such therapies at the time of their last clinical appointment. Overall, 87% of all youth received some type of psychotherapy targeting depression, with the lowest use of psychotherapy being among those with IDD (71%, P = 0.167, FET). These data are included in Figure 2. Intensive in-home services were more commonly used for youth with IDD (29%, P 0.029, FET).
Discussion
In the absence of practicable evidence-based guidelines for treating depression in youth with ASD and IDD, this study reveals novel information about current prescribing practices for depression among youth with ASD or other IDDs. Notably, 11% of sampled individuals were prescribed no medication for depression. Reasons for no depression medication use were multifactorial and included issues of poor medication tolerability, parental preference, and prescriber access (for those traveling from rural areas for one-time assessments). Consistent with prior data for children with ASD, antidepressants, in particular SSRIs, were the most commonly prescribed medication class [8,13]. Specifically, escitalopram, fluoxetine, and sertraline were prescribed most often. This is in spite of evidence to suggest SSRI’s may not be well-tolerated in youth with ASD and warrants further research.
General historical prescribing practices in youth with ASD have been well recorded and highlight significant issues concerning polypharmacy. In a previous study of 33,565 children with ASD by Esbensen et al. (2009), 64% were prescribed one psychotropic medication, 35% were prescribed two or more classes of psychotropic medication, and 15% were prescribed medications from three or more classes. Several of our findings align with such studies and suggest that our treatment algorithms for depression are ineffective for both youth with ASD and IDD. Given that children with ASD and/or other IDDs were not included in the original trials of antidepressants in pediatric cohorts, this is unsurprising though again sheds light on the critical need for further research. Eight percent of all individuals were on either a mood stabilizer or antipsychotic for depression rather than an antidepressant. Of those prescribed medication for depression, 39% were on two or more medications specified for depression. Antipsychotic augmentation of depression was prescribed for 17% of those on antidepressants. Our study highlights the unique difficulties in treating those with both ASD and IDD, as these youth demonstrated higher prevalence of polypharmacy, antipsychotic use, and depression augmentation.
Underlying reasons for pharmacologic treatment differences, including polypharmacy, in youth with ASD (as well as IDD) include poor diagnostic clarity with vague symptoms as primary drivers of medication selection. This has perpetuated a poor understanding of best-practice pharmacologic treatment of formally diagnosed depression in youth with ASD, and despite more recent inclusion of children with IDD in clinical trials; the evidence base remains woefully lacking. As a result, providers are left to attempt to pharmacologically manage depression in the ASD population on an uncoordinated, uninformed, and trial-and-error basis. Our findings highlight issues both of undertreatment and polypharmacy, which may be related to poor tolerability and poor response as suggested by the need for several medication trials, particularly in those with ASD and IDD. The data gleaned from this study will guide the design of prospective pharmacologic trials for the treatment of depression in youth with ASD and/or IDD.
Another area for future study is the use (or lack thereof) of psychotherapy for youth with IDD. Overall, less youth in this study were receiving psychotherapy services than *** (would make argument why this feels important–is is less than neurotypical youth? Less than you’d want to see for the depression diagnoses but not necessarily less than neurotypical youth?) We hypothesize that this trend is related to the lack of evidencebased modalities for depression treatment and a dearth of supportive resources for those with IDDs in comparison to those with ASD. Future studies of depression treatment in ASD and IDD should ideally include a therapy-only treatment arm. Further, if currently offered treatment modalities are not appropriate for neurodiverse youth, we must prioritize research of novel psychotherapeutic treatments for mood and anxiety disorders in youth with concurrent ASD or IDDs.
Strenths and Limitations
The current study has several notable strengths. To our knowledge, this study is the first to describe the naturalistic pharmacologic approaches taken in an outpatient clinical setting specifically for depression in children and adolescents with ASD. Additionally, the charts reviewed were for patients who were seen in clinic over a five-year period by numerous providers, generating a longitudinal record of diverse prescribing practices and accompanying clinical reasoning.
There are a number of limitations that must be considered when interpreting our study results. Our study was limited by the amount and quality of information in patient charts. Data quality was notably limited by inconsistent use of correct ICD codes, limited documentation of presenting symptoms in chart notes, and the possibility of incorrect medication lists. Additionally, our study features prescribing practices of psychiatrists affiliated with a specific institution. There may be geographical differences in prescribing practices that the present study failed to capture, and future studies may benefit from including data from numerous clinic sites.
Conclusion
By conducting and promoting studies examining current treatment practices, the scientific community moves to close the gap in knowledge and quality of care that our neurodiverse youth often face when seeking treatment for depression. It is critical that we develop a more complete understanding of the nature and common behavioral phenotypes of depression in youth with ASD and subsequently identify the treatment modalities that are the safest and most effective in this cohort. Children and adolescents with ASD are a medically vulnerable population with a unique set of characteristics, needs, nuances, and presentations, and we cannot continue to assume that the accepted standard of treatment in neurotypical youth applies to the ASD population in the same way. It is our professional duty to continue to advocate for this population through continued research and improvement in the standards of treatment and care.
Acknowledgements: Grant for funding and statistical support by the North Carolina Translational and Clinical Sciences Institutes. Grant number: CTSA—UL1TR002489.
References
- Hudson CC, Hall L, Harkness KL. Prevalence of Depressive Disorders in Individuals with Autism Spectrum Disorder: a Meta-Analysis. Journal of Abnormal Child Psychology. 2019; 47: 165-175. https://doi.org/10.1007/s10802-018-0402-1.
- NIMH. Major Depression. The National Institute of Mental Health Information Resource Center. 2019; Retrieved November 18, 2019 from https://www.nimh.nih.gov/health/statistics/ major-depression.shtml
- Centers for Disease Control and Prevention Data and statistics on children’s mental health. The Center for Disease Control and Prevention. 2019. Retrieved from https://www.cdc.gov/childrensmentalhealth/data.html
- Nah YH, Brewer N, Young RL, Flower R. Brief Report: Screening Adults with Autism Spectrum Disorder for Anxiety and Depression. Journal of Autism and Developmental Disorders. 2018; 48: 1841-1846. https://doi.org/10.1007/s10803-017-3427-3
- Sterling L, Dawson G, Estes A, Greenson J. Characteristics associated with presence of depressive symptoms in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2008; 38: 1011-1018. https://doi.org/10.1007/ s10803-007-0477-y
- Chandrasekhar T, Sikich L. Challenges in the diagnosis and treatment of depression in autism spectrum disorders across the lifespan. Dialogues in Clinical Neuroscience. 2015; 17: 219-227. https://doi.org/10.31887/DCNS.2015.17.2/tchandrasekhar
- Fung LK, Mahajan R, Nozzolillo A, Bernal P, Krasner A, et al. Pharmacologic Treatment of Severe Irritability and Problem Behaviors in Autism: A Systematic Review and Meta-analysis. Pediatrics. 2016; 137: S124-135.
- Houghton R., Ong RC, Bolognani F. Psychiatric comorbidities and use of psychotropic medications in people with autism spectrum disorder in the United States. Autism Research. 2017; 10: 2037-2047. https://doi.org/10.1002/aur.1848
- March J, Silva S, Petrycki S, Curry J, Wells K. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment For Adolescents With Depression Study (TADS) randomized controlled trial. Journal of the American Medical Association. 2004; 292: 807-820. https://doi. org/10.1001/jama.292.7.807
- King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: Citalopram ineffective in children with autism. Archives of General Psychiatry. 2009; 66: 583-590. https://doi.org/10.1001/archgenpsychiatry.2009.30
- Williams K, Brignell A, Randall M, Silove N, Hazell P, et al. Selective Serotonin Reuptake Inhibitors (SSRIs) for Autism Spectrum Disorders (ASD). The Cochrane Database of Systematic Reviews. 2013; 8: CD004677. https://doi.org/10.1002/14651858. CD004677.pub3
- Persico AM, Ricciardello A, Lamberti M, Turriziani L, Cucinotta F, Brogna C, Vitiello B, Arango C, et al. The pediatric psychopharmacology of autism spectrum disorder: A systematic review - Part I: The past and the present. Prog Neuropsychopharmacol Biol Psychiatry; 2021; 110: 110326.
- Esbensen, AJ, Greenberg JS, Sletzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009; 39:1339–1349. https://doi.orgo/10.1007/s10803-009-0750-3