Research Article
Volume 2, Issue 1
Evaluation of the Earliest Time for Induction of Abortion and Progesterone Profile Changes by Dexamethasone Injection in the Pregnant Heifers
Hossein Hamali1*; Javad Jafari2; Ezzatullah Fathi1
1Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Tabriz, Iran.
2Department of Theriogenology, Faculty of Veterinary Medicine, University of Tehran, Iran, and Graduated of Veterinary Medicine, University of Tabriz, Iran.
Corresponding Author :
Hossein Hamali
Email: hamali@tabrizu.ac.ir
Received : Dec 20, 2022 Accepted : Jan 16, 2023 Published : Jan 23, 2023 Archived : www.meddiscoveries.org
Citation: Hamali H, Jafari J, Fathi E. Evaluation of the Earliest Time for Induction of Abortion and Progesterone Profile Changes by Dexamethasone Injection in the Pregnant Heifers. Med Discoveries. 2023; 2(1): 1009.
Copyright: © 2023 Hamali H. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The use of dexamethasone in many diseases such as ketosis, drug and food allergies and septic shock is pointed out in cows. Dexamethasone use in pregnant cows is limited due to it, s abortive effect. On the other hand, dexamethasone does not always cause abortion. So the aim of our study was to detect the exact period that we can use dexamethasone in pregnant heifers. To achieve this purpose, we selected 40 Holstein heifers from farms with the same nutritional and welfare systems. After confirmation of conception with ultrasonography 30 days after mating, Holstein heifers were divided into two completely randomized groups. In group A (n = 20, treatment), 30 mg dexamethasone was administered intramuscularly to the cows every 15 days until the heifers started abortion. In group B (n = 20, control) 15 cc of normal saline was injected every 15 days. Blood samples were taken 24 hours before the injection and 72 hours after the injection then progesterone levels were measured. The exact time of reduction in progesterone levels in the blood was recorded. The results were analyzed by using Chi-Square statistical methods (on the rate of abortions) and t-tests on progesterone changes before and after dexamethasone injection and using software version 22, Inc., Chicago, IL, USA SPSS. Based on our findings, dexamethasone’s first effect was at 75 days of pregnancy (p < 0.05), so it can be used up to day, s 75 of pregnancy. Dexamethasone’s effect can be attributed to changes in the concentration of progesterone, estrogen and the number of their receptors during pregnancy.
Keywords: Heifer; Progesterone; Abortion; Pregnancy; Dexamethasone.
Introduction
Dexamethasone is associated with many limitations due to abortion in pregnant cows and there is no exact information about the first time dexamethasone had an effect on abortion in pregnant cattle. Despite extensive studies on the consequences of increasing synthetic glucocorticoids in mid- and late pregnancy, relatively few studies have been conducted on the importance of synthetic glucocorticoids in early pregnancy. Existing studies show that the use of synthetic glucocorticoids in early pregnancy is controversial. Previous studies have shown that synthetic glucocorticoids can have both positive and negative effects in early pregnancy [1]. Synthetic glucocorticoids exert a wide range of positive effects in early pregnancy, such as suppressing Natural Killer cells (NK) and stimulating chorionic gonadotropin secretion, as well as increasing trophoblast proliferation and invasion. However, synthetic glucocorticoids can cause a range of side effects, such as placental apoptosis and impaired nutrient transfer from the placenta, which prevents pregnancy from continuing [2]. In pregnant animals with pregnancy problems, glucocorticoids such as dexamethasone, betamethasone, prednisolone, and methylprednisolone may be used for preterm delivery and inflammatory conditions during pregnancy [3,4]. Despite the widespread use of dexamethasone in veterinary medicine, some pregnancy-related side effects, including miscarriage, have been reported in some animals. The mechanism of this type of response is not clear, but it seems that this mechanism is related to differences between species or races in response to changes in the concentration of some reproductive hormones, including progesterone [5]. Broussard JR, et al. [6] injected dexamethasone into non-pregnant cows and found that it delayed follicular growth by prolonging the estrous cycle by 10 days. In other words, the estrous cycle is 31 days instead of 21 days. Regarding estrogen and progesterone concentrations, it was concluded that dexamethasone injection had no effect on progesterone concentrations in non-pregnant cows but significantly reduced the concentration of estradiol in the blood of these cows. In 2011, Duong HT, et al. [7] examined the effect of cortisol on pregnant heifers and concluded that cortisol in the early stages of pregnancy supported the formation of the corpus luteum and increased progesterone secretion, supporting the early embryo in the implantation line and ultimately increasing the pregnancy rate compared to the control group showed that after 20 mg dexamethasone injection, luteolysis occurs between 1.6 and 0.4 days before delivery, and delivery itself occurs about 7 days after dexamethasone injection [4]. Hamali H, et al. [8] showed that dexamethasone injection alone induces calving in heifers 42.5 hours after injection, while injection of dexamethasone with estradiol induces calving in heifers 37.5 hours later. Yahi D, et al. [5] showed that dexamethasone injection has no effect on the concentration of progesterone and estradiol in goats’ blood, so dexamethasone can be used in pregnant goats without adverse effects.
Materials and methods
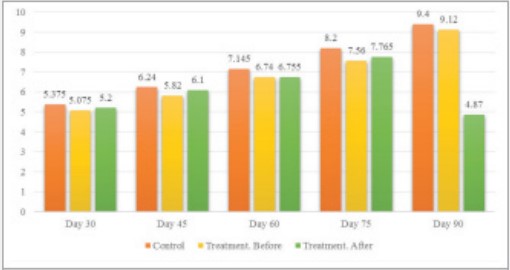
Heifers were thoroughly examined for overall health and fetal health. Heifers that were not diagnosed as healthy and were suspected of having ionic disease, BVD/MD, chronic diarrhea, chronic pneumonia, severe lameness, and severe weight loss were excluded from the study. For this purpose, 40 pregnant Holstein heifers were selected from farms around Tabriz that had similar conditions in terms of nutrition and health, and after confirmation of pregnancy by ultrasound on the 30th day after mating were placed in group A (n = 20, treatment) . According to the breeders, these heifers had inadvertently mated and due to possible problems during calving, it was necessary to implement a termination program for them. Twenty 30-day-old pregnant heifers were selected from the same farms as the control group after confirmation by ultrasound and placed in group B (n = 20, control). In group A, 30 mg of dexamethasone (15 ml and 2% vetacoid manufactured by Abureihan Pharmaceutical Company) was administered intramuscularly every 15 days until the heifers started aborting. After prescribing the drug, farmers were advised to immediately inform the veterinarian in case of any abortion, otherwise record the exact time of the abortion. After tying and beating the nasal catheter to the heifers, 6 ml of blood was aspirated from the jugular vein to a 10 ml syringe and transferred to a 10 ml test tube. The samples were immediately taken to the laboratory and to separate the serum, the tubes containing blood were centrifuged at 3500 rpm for 10 minutes. The isolated sera were then transferred to a numbered microtube and stored in the refrigerator until the experiment was performed. Measurement of progesterone was performed by using an ELISA kit (Immunolab GmbH, Germany). The results were analyzed by using Chi-Square statistical methods (on the rate of abortions) and t-tests on progesterone changes before and after dexamethasone injection and using software version 22, Inc., Chicago, IL, USA SPSS. RESULTS Progesterone concentration was measured in heifers of the treatment group before and after dexamethasone injection and also in heifers of the control group (Table 1). As can be seen, the concentration of progesterone in the peripheral blood plasma increases with the progression of pregnancy and dexamethasone injection until the 90th day or 3rd month of pregnancy has no effect on reducing the concentration of plasma progesterone. After injecting dexamethasone at 90 days of age, the heifers of the treatment group aborted 13 heifers at an interval of 56.3 hours (average) (Figure 1). This suggests that after 75 days of age, cows gradually became able to convert progesterone to estrogen under the influence of corticosteroids, with more than 50% of them having an abortion 90 days after dexamethasone injection. In other words, 75 days after the onset of pregnancy is the last time that dexamethasone injection does not cause miscarriage, and after this stage, the risk of miscarriage due to dexamethasone injection is greatly increased. There was no statistically significant difference between the control group and the treatment group before and after dexamethasone injection in terms of blood progesterone concentration, except for the treatment group on day 90, where 13 heifers had abortions. Their mean progesterone blood concentration dropped from 9.2 ng/mL to 2.7 ng/ mL. In this case, the statistical difference was quite significant (p ≤ 0.01). Dexamethasone injection resulted in a significant difference (p < 0.05) between control and treatment groups at 60, 75 and 90 times. At 45 and 90 times, a significant difference was observed in the treatment group before and after injection (Table 2, Figure 2).
Discussion and conclusion
The results of this study showed that the effect of dexamethasone on abortion in cows is a time-dependent effect. In other words, a pair of cows must reach a stage of growth and maturity that can convert the progesterone production line in the placenta to estrogen under the influence of dexamethasone. Contrary to previous studies [9] which believed that dexamethasone affects the placenta at 5 months of age, the results of this study showed that dexamethasone is around the third month or 90 days of age that affect the placental hormone production process and causes abortion.
Table 1: Mean progesterone concentration in heifers’ blood in control and treatment groups before and after dexamethasone injection (ng/mL).
| Control Group | Treatment Group (Before injection) | Treatment Group (72 after injection) | Pregnancy Time (Day) |
|---|---|---|---|
| 5.4 | 5.1 | 5.2 | 30 |
| 6.3 | 5.9 | 6.1 | 45 |
| 7.1 | 6.8 | 6.9 | 60 |
| 8.2 | 7.7 | 7.8 | 75 |
| 9.4 | 9.2 | 8.9 | 90 |
| 10.3 | 9.2 | 2.7 | 90 |
Table 2: Mean blood progesterone concentration at different times and groups.
| Time | Groups | N | MEAN 1 STD. DEVIATION |
|---|---|---|---|
| Control | 20 | 5.3750 0.40 | |
| R30 | Before Treatment | 20 | 5.0750 0.17 |
| After Treatment | 20 | 5,2000 0.26 | |
| Total | 60 | 5.2167 0.31 | |
| Control | 20 | 6.2400 0.29 | |
| R45 | Before Treatment | 20 | 5.8200 0.44 |
| After Treatment | 20 | 6.1000 0.22 | |
| Total | 20 | 6.0533 0.37 | |
| Control | 20 | Control | |
| R60 | Before Treatment | 20 | 6.7400 0.49 |
| After Treatment | 20 | 6.7550 0.44 | |
| Total | 60 | 6.8800 0.44 | |
| Control | 20 | 8.2000 0.31 | |
| R75 | Before Treatment | 20 | 7.5600 0.44 |
| After Treatment | 20 | 7.5600 0.44 | |
| Total | 60 | 7.8417 + 0.49 | |
| Control | 20 | 9.4000 0.25 | |
| R90 | Before Treatment | 20 | 9.1200 0.43 |
| After Treatment | 20 | 4.8700 + 3,045 | |
| Total | 60 | 7.7967 + 2,727 |
Interestingly, based on studies conducted in this study, it was found that the decrease in progesterone concentration in the blood of cows occurs about 24 hours after injection of dexamethasone, but abortion occurs about 56 hours after injection of dexamethasone. Due to the significance of the chisquare test, dexamethasone injection leads to miscarriage. Due to the significant differences between the control and treatment groups at times 60, 75 and 90, dexamethasone injection leads to a significant reduction in progesterone levels at times 60, 75 and 90. Dexamethasone injections at 45 and 90 times resulted in a significant difference (severe decrease in progesterone levels) in the treatment group. However, according to previous studies by Hamali H, et al. [8], injecting dexamethasone into heifers 270 days aft er inoculation resulted in preterm delivery within 43 hours. This suggests that as the fetus ages, the placenta becomes more capable of synthesizing estradiol and causing miscarriage. The study by Yahi D, et al. [5] showed that the concentration of progesterone and estrogen in dexamethasone treatment during pregnancy in goats was not significantly affected. The results in this study are similar to those of Ohrlander S, et al. [10], who reported that dexamethasone did not alter serum progesterone levels to induce fetal lung maturation in humans, but differed from that of Ahmadabad HN, et al. [11] they reported that progesterone levels decreased in dexamethasone-treated pregnant mice. Th e observed diff erences can be attributed to species diff erences according to the source of progesterone secretion during pregnancy. Progesterone is mainly produced by the Corpus Luteum (CL) and the placenta, and to a lesser extent by the adrenal cortex during pregnancy in most mammals. Unlike mice, in goats, progesterone is secreted mainly by the corpus luteum during pregnancy (low dependence or lack of dependence on placental participation). Despite the widespread clinical use of dexamethasone, it has been reported to cause side eff ects on the placenta, including weight loss, placental size, and placental effi cacy in some animal models as well as humans. Th erefore, since in goats, progesterone is mainly produced by the corpus luteum during pregnancy, the possible side eff ects on placental progesterone production in goats are of little importance. Th is may be due to the ineff ectiveness of the drug dexamethasone in the secretion of corpus luteum progesterone despite the adverse eff ects on the placenta in goats. On the other hand, the lack of eff ect of dexamethasone on estrogen levels during pregnancy in goats is contrary to a previous report by Ylikorkala O, et al. [12], and Ahmedabad HN, et al. [11]. Find the lack of eff ect of dexamethasone on estrogen concentration in this study indicates that dexamethasone has no negative eff ect on estrogen-producing and estrogen-promoting structures such as the ovaries and adrenal glands. Regulation of progesterone receptors in goat uterus by dexamethasone observed in this study can be one of the benefi cial eff ects of dexamethasone in trying to increase progesterone sensitivity. McDonald TJ, et al. [13] reported that glucocorticoids are involved in the heterologous regulation of several hormone receptors. Th is mechanism is probably through the regulation of mRNA receptor levels by aff ecting the increase in PR mRNA levels and gene transcription reported by Kraus and Katzenellenbogen in rats. Th erefore, dexamethasone may stimulate PR transcriptional activity and increase PR expression in utero. Weight loss at birth may be due to decreased uterine exchange, which may be due to placental weight loss or placental function.
In a study by Ohrlander S, et al. [10] on the outcome of corticosteroid therapy in late pregnancy, levels of Estrone (E1), Estradiol-17beta (E2) and progesterone were observed in maternal plasma and amniotic fluid before and after treatment. At 35-30 weeks of gestation, 12 mg of betamethasone was injected daily for 3 days to prevent Idiopathic Respiratory Distress Syndrome (IRDS) and was compared with the control group. Steroid concentrations were determined using radioimmunoassay. Plasma E1 and E2 levels decreased by 38% and 29%, respectively, while progesterone was not affected. No significant change in the concentration of steroids in the amniotic fluid was observed. According to a study by Vighio GH, et al. [14], the effect of glucocorticoids on the concentration of gonadal and gonadotropic steroid hormones and subsequent follicular activity in cows undergoing normal estrous cycle was evaluated using dexamethasone in the middle of the luteal phase. Seven cows were injected with saline twice a day from day 13 to day 17 of the estrous cycle (control). During the next bovine cycle, dexamethasone (2 mg, IM) was injected twice daily on days 13 to 17. Plasma samples were measured twice a day from the control and treatment groups for progesterone and estradiol concentrations. The onset of estrus after dexamethasone treatment was delayed by up to 3 days and from day 23 to 25 in 3 cows and was not observed on day 4 in 35 cows compared to the mean cycle length of 22.4 3. 3.2 in control cows. Progesterone concentrations remained significantly (p < 0.01) on days 19 to 23, while estradiol levels could not increase (p < 0.05) on days 19 and 20 aft er dexamethasone treatment. Compared with the values in the control group, there was a significant decrease in the pulse of Luteinizing Hormone (LH) and estradiol (p < 0.05), although the number of hormone pulses per 12 hours in dexamethasone treated cows was not affected. Baseline concentrations of LH and estradiol did not change according to the type of treatment. Braun T, et al. [15] suggest that early treatment with dexamethasone is associated with specific changes in BNC counts and may also be associated with markers of placental apoptosis, which are associated with changes in the placenta and fetal development. The data presented may indicate that initial dexamethasone treatment in sheep, which is associated with specific changes in placental development, BNC number, and function, may be associated with short- and long-term changes in fetal growth and endocrine axis. The development of the fetus during pregnancy and its function depends on the capacity of the placenta according to the wishes of the fetus. As confirmed in the present study, fetal weight is related to placental weight. Early exposure to dexamethasone results in a decrease in fetal weight and a change in placental growth, some of which lasts until delivery. Placental distribution and size can be affected by adverse intrauterine conditions. The presence of a large number of placentas in groups C and D [15] has been suggested as a placental adaptation to increase nutrient uptake into the fetus. We have shown that changes in placenta ratios and expression of important placental enzymes (prostaglandin G/H synthetase 2) occurred after GC treatment in late pregnancy. Initial treatment with dexamethasone in females in the present study did not significantly change placental weight or the number of placental subtypes compared to the control group but increased the mean weight gain of subgroup C pairs compared to the control group. May indicate placental compatibility with initial dexamethasone treatment. The unique ruminant trophoectoderm produces BNCs, and after cell maturation and migration to the maternal-embryonic part, the BNCs combine with the maternal epithelium to form the placenta. Bipolar cells make up 10% to 20% of sheep fetal trophoctoderm cells and their main function is to transfer embryonic hormones (glycoproteins associated with pregnancy and prolactin), regulate the intrauterine environment, and meet fetal needs. Sheep placental lactogen plays an important role in fetal growth by affecting maternal metabolism and regulating fetal access to nutrients. The seeds containing placental lactogen are transferred to the fetal-maternal part and enter the maternal and fetal circulation. Variation in fetal weight was associated with placental weight, maternal serum lactogen, and lactogen-related mRNA concentration. In sheep, glucocorticoid exposure in late pregnancy resulted in a significant reduction in birth weight, which was associated with a decrease in the mean number of BNCs, placental lactogen, and maternal and fetal lactogen levels. In the present study, treatment with dexamethasone on day 100 of pregnancy resulted in temporary fetal weight loss (in female fetuses only). This decrease in female fetal weight was associated with a decrease in BNC counts but no change in placental lactogen protein level. The main mechanism of the decrease in the number of BNCs and placental lactogen aft er dexamethasone treatment has not yet been elucidated, but may be associated with an increase in apoptotic agents and thus an increase in the rate of BNC apoptosis. The mRNA expression level of placental proapoptotic markers (Caspase-3 on day 100 of gestation and p53 on day 125) increased and anti-apoptotic markers (PCNA on day 100 of gestation) decreased significantly compared to the control group. Glucocorticoid-induced apoptosis may not be dependent on external pathways. We know that our study is not without limitations. The placenta may have changes that can be seen at the level of proteins or mRNA. However, this is difficult because of the extensive interaction between maternal and fetal tissue, and placental staining can be a better sign of changes in fetal and maternal tissue. Our knowledge of placental lactogen protein levels allows us to relate the effects of dexamethasone on the number of BNCs to their performance. We have shown that there is no correlation between fetal weight and placental lactose levels at 100 days of gestation, but dexamethasone disrupts normal communication between the maternal and fetal units of the placenta. On day 125 of gestation, fetal size was normal in females in the dexamethasone treatment groups, with an average normal BNC count, and significantly increased OPL-placental protein levels. The weight of male embryos in both control and dexamethasone-treated groups depended on placental weight, BNC number, placental plasma protein and maternal OPL plasma level. In males, early treatment with dexamethasone did not significantly alter fetal growth compared with controls. However, on day 50 of gestation, placental OPL protein levels increased significantly compared with controls (most prominent in subgroup A), which may indicate a rapid BNC production response. No changes were observed in apoptotic and anti-apoptotic markers, which may indicate placental adaptation in males. Male embryos appear to adapt their placental function to maintain continued growth, but female embryonic growth slows. Accordingly, we found that male fetal weight was not affected by initial treatment with dexamethasone. In contrast, females showed temporary adaptation to dexamethasone treatment with dexamethasone treatment, especially in relation to placental distribution and function, fetal HPA axis activity, and postnatal endocrine response.
References
- Boomsma CM, Kamath MS, Keay SD, Macklon NS. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst Rev. 2022; 6: CD005996.
- Gur C, Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol. 2004; 18: 93-101.
- Kask K, Gustafsson H, Gunnarsson A, Kindahl H. Induction of parturition with prostaglandin f2 alpha as a possible model to study impaired reproductive performance in the dairy cow. Anim Reprod Sci. 2000; 59: 129-139.
- Königsson K, Kask K, Gustafsson H, Kindahl H, Parvizi N. 15-ketodihydroPGF2 alpha, progesterone and cortisol profi les in heifers after induction of parturition by injection of dexamethasone. Acta Vet Scand. 2001; 42: 151-159.
- Yahi D, Ojo NA, Mshelia GD. Effects of dexamethasone on progesterone and estrogen profiles and uterine progesterone receptor localization during pregnancy in Sahel goat in Semi-Arid region. J Anim Sci Technol. 2017; 59: 12.
- Broussard JR, Rocha A, Sirois J, Roussel JD, Thibodeaux JK, et al. Effects of dexamethasone administration to diestrus cows on systemic progesterone, estrogen and uterine cyclooxygenase production. Anim Reprod Sci. 1997; 47: 263-271.
- Duong HT, Piotrowska-Tomala KK, Acosta TJ, Bah MM, Sinderewicz E, Majewska M, Jankowska K, Okuda K, Skarzynski DJ. Eff ects of cortisol on pregnancy rate and corpus luteum function in heifers: an in vivo study. J Reprod Dev. 2012; 58:223-230.
- Mehrvar N, Hamali H, Saberivand A. Comparison of two different protocols for induction of parturition in heifers with or without estradiol benzoate. Journal of Life Sciences. 2017; 11: 30-34.
- Purohit GN, Shekher C, Kumar P, Solanki K. Induced termination of pregnancy in domestic farm animals. Iranian Journal of Applied Animal Science. 2012; 2: 1-12.
- Ohrlander S, Gennser G, Batra S, Lebech P. Effect of betamethasone administration on estrone, estradiol-17 beta, and progesterone in maternal plasma and amniotic fluid. Obstet Gynecol. 1977; 49: 148-153.
- Namdar Ahmadabad H, Kayvan Jafari S, Nezafat Firizi M, Abbaspour AR, Ghafoori Gharib F, et al. Pregnancy outcomes following the administration of high doses of dexamethasone in early pregnancy. Clin Exp Reprod Med. 2016; 43: 15-25.
- Ylikorkala O, Dawood MY, Kauppila A, Tuimala R. Effect of maternal dexamethasone therapy on the levels of oestrogens, progesterone and chorionic gonadotrophin in amniotic fluid and maternal serum. Br J Obstet Gynaecol. 1978; 85: 334-337.
- McDonald TJ, Franko KL, Brown JM, Jenkins SL, Nathanielsz PW, Nijland MJ. Betamethasone in the last week of pregnancy causes fetal growth retardation but not adult hypertension in rats. J Soc Gynecol Investig. 2003 Dec; 10(8):469-73. doi: 10.1016/s1071-5576(03)00151-5. PMID: 14662159.
- Vighio GH, Liptrap RM. Plasma hormone concentrations after administration of dexamethasone during the middle of the luteal phase in cows. Am J Vet Res. 1990; 51(11):1711-1714.
- Braun T, Meng W, Shang H, Li S, Sloboda DM, Ehrlich L, et al. Early dexamethasone treatment induces placental apoptosis in sheep. Reprod Sci. 2015; 22: 47-59.