Review Article
Volume 2, Issue 9
Homeostasis of Circadian Rhythm and Therapeutic Approaches to Manage its Related-Disorders: The Case of Medicinal Mushrooms Rich in Melatonin
George D Tselepis; Ioannis S Palamiotis; Sofia K Georgiou-Siafis*
Faculty of Health and Rehabilitation Sciences, Metropolitan College, 41005, Larissa, Greece.
Corresponding Author :
Sofia K Georgiou-Siafis
Email: sgeorgiou@mitropolitiko.edu.gr
Received : Aug 20, 2023 Accepted : Sep 18, 2023 Published : Sep 25, 2023 Archived : www.meddiscoveries.org
Citation: Tselepis GD, Palamiotis IS, Georgiou-Siafis SK. Homeostasis of Circadian Rhythm and Therapeutic Approaches to Manage its Related-Disorders: The Case of Medicinal Mushrooms Rich in Melatonin. Med Discoveries. 2023; 2(9): 1075.
Copyright: © 2023 Georgiou-Siafis SK. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Circadian rhythm consists of complicated molecular mechanisms to orchestrate environmental stimuli to intrinsic molecular clocks. However, circadian rhythm is frequently deregulated in modern society by both extrinsic and biological factors leading to Circadian Rhythm Sleep Disorders (CRSD). Superchiasmatic nucleus in hypothalamus is the central oscillator of circadian rhythm via both receiving light information from retina and sending chemical signals to peripheral clocks. Multiple molecular mechanisms fluctuate the expression of around half of the genome, as shown in mice, during day-time. Brain and Muscle RNT-Like 1 (Bmal)/ Circadian Locomotor Output Cycles Kaput (Clock) is the major transcriptional complex, acting on reprogramming cells, via transcriptional-translational loops. Upon low external light, pineal gland secretes melatonin to promote sleep, via its MT1 and MT2 receptors. Deregulation of the circadian rhythm causes both short term and long term effects, including on metabolism, and female reproductive system. Melatonin is the gold standard drug used for CRSD, but it is characterized by a short half-life, and performs well, in work shifters and patients suffering the Delayed Sleep Phase Type (DSPS) syndrome. Novel melatonergic drugs have been developed, with a partial increase in half-life and bioavailability. Medicinal mushrooms are a rich source of melatonin, used in traditional medicine for insomnia and have been tested for depressive disorders. In the current review, we discuss the alternative use of medicinal mushrooms, enriched in melatonin (as the Hericium erinaceus, Lactarius deliciosus and Armillaria mellea), for the treatment of CRSDs. We conclude that there is a need for more studies to unravel the potential value of medicinal mushrooms in the upcoming field of ‘’drugging the clock’’ therapeutic approaches.
Keywords: Circadian rhythm sleep disorders; Melatonin; Medicinal mushrooms; Circadian rhythm homeostasis; Therapeutic interventions.
Abbreviations: AA-NAT: arylalkylamine-N-acetyltransferase; ASPS: Advanced Sleep Phase Type; Bmal1: Brain and Muscle RNT-Like 1; Clock: Circadian Locomotor Output Cycles Kaput; CRSD: Circadian Rhythm Sleep Disorder; Cry1: Cryptochrome Circadian Regulator 1; DSPS: Delayed Sleep Phase Type; EFSA: Europeans Food Safety Authority; GABA: gamma-amino butyric acid; ipRGCs: intrinsically photoreceptive retinal ganglion cells; MT1/2: Melatonin receptors; Per1: Period Circadian Regulator 1; PVN: paraventricular nucleus; SCN: suprachiasmatic nucleus; TF: Transcriptions factor.
Introduction
The biological mechanisms of organisms tend to be synchronized by environmental changes; the major influence is the continuous rotation of earth, through its axis, creating a 24 hr light-dark cycle. The circadian rhythm is an endogenous system, consisting of oscillators and dictating body homeostasis. It is found in almost every organism, including animals, plants, fungi, and in some bacteria (cyanobacteria) [1]. The first scientific proof of circadian rhythm was made in 1729, by the French astronomer Jean Jacques d’ Ortous de Mairan. He examined the plant mimosa by exposing it to a light-tight dark room and claimed that the plant unfolds its leaves in the morning and closes them in the evening [2].
In multicellular organisms, such as mammals, it is difficult for the tissues to get photic information. The latter is received by the retina and transmitted to the hypothalamus where the Superchiasmatic Nucleus (SCN), that serves as a central oscillator orchestrates the information to the peripheral tissues. Central to this pathway are the intrinsically photoreceptive Retinal Ganglion Cells (ipRGCs) which are involved through the retinohypothalamic tract [3]. The pathways used by SCN to relay temporal information to the tissues comprise of complex signals, divided into four categories: sympathetic and parasympathetic nervous system, hormonal outputs, feeding-fasting rhythms, and recurrent change of core temperature [4].
During the 24 h light-cycle, a remarkable alteration in the expression pattern occurs through auto-regulatory loops, transcriptional and translational mechanisms [4]. A detailed transcriptomic analysis (circadian clock atlas) conducted in 2014 by Zhang R. et al, show that 43% of the mouse genes, at multiple organs, fluctuate their expression during the daytime and night [5]. It is interesting to note, that the products of these circadian-regulated genes are molecular targets of many commonly prescribed drugs. In particular, the transcriptional initiation is a key step, through the coordination conducted by the Circadian Locomotor Output Cycles Kaput (Clock)/Brain and Muscle RNTLike 1 (Bmal1) transcription factors (TF) [6] (discussed below). Epigenetic regulations leading to reprogramming are also an important event in circadian rhythm expression fluctuations. For example, Clock acts itself as a histone acetyl-transferase [7]. Post-transcriptional effects regulate the stability of the clockassociated genes [8], such as miRNA-219 and -132 targeting Clock/Bmal1 TF [9]. Moreover, post-translational modifications, particular phosphorylation, govern the circadian cycle oscillations [10].
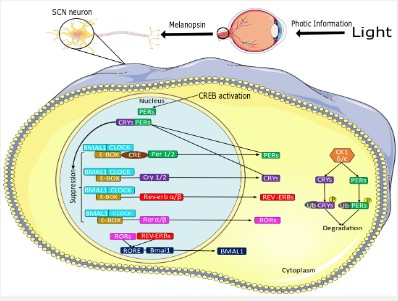
Photic information triggers the ipRGCs via the blue light-sensitive vitamin A-based photopigment melanopsin (OPN4) [11]. After that, the information comes across the retinohypothalamic tract as an electric signal and then it is transformed into chemical information through the release of glutamate in the SCN. Here, increase in intracellular cAMP activates the cAMP Response Element Binding Protein (CREB) inducing Period Circadian Regulator 1 (PER1), PER2, Salt Inducible Kinase 1 (SIK1) [11] (Figure 1). In both SCN and peripheral clocks, the circadian mechanisms involve cell-autonomous transcriptional-translational feedback loops [11]. The initial event is the binding of the major circadian rhythm TF Clock/Bmal1 to the promoter elements E-box, where multiple TF are induced: Per1, Per2, Per3, Cryptochrome Circadian Regulator 1 (Cry1) and Cry2. The transcriptional activity of Clock/Bmal1 heterodimer is high in the morning, while suppressed during nighttime, by the accumulation of its products Per/Cry (Negative feedback loop). Per and Cry are regulated by post-translational modifications, such as phosphorylation by Casein Kinase 1δ and ubiquitination, leading to their degradation, which signals the onset of a new circadian cycle.
Furthermore, another interlocked loop that regulate the expression of Per and Cry proteins is dependent on nuclear receptor subfamily 1 group D member 2 (Nr1d2 or Rev-erbα/β) TF, that regulate the Bmal1 promoter via ROR elements [4]. As photic information is positive, the SCN secrets gamma-amino butyric acid (GABA), responsible to suppress melatonin secretion by the pineal gland [12].
Light reaches to ipRGCs of the retina and through the neurotransmitter glutamate signals to SCN the increase of cAMP. This increases CREB-dependent transcriptional activation of Per proteins, leading to the advancing of the molecular clockwork [11]. Per proteins are short-lived due to post-translational modifications, and thus this stimulus is short-term. The main Clock/Bmal TF complexes are presented, via binding to E-boxes of several target genes. Per and Cry proteins act as negative regulators of Clock/ Bmal, but the former levels decline at the dawn, so a next circadian cycle begins, each next morning. Bmal levels are in antiphase to the levels of Cry and Per. Bmal levels peak at dawn through the regulation of Ror TF. Some components of the figures were acquired from Servier Medical Art
Sleep and melatonin
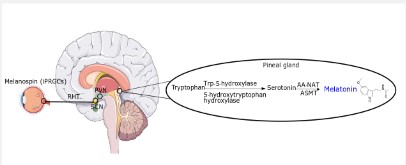
Sleep, crucial for overall human health, is promoted by the hormone melatonin, when the light intensity is low (<30 lux, dim light), at a wavelength of 555 nm (red) [13]. The information gets to the melanopsin of retinal ganglion cells again, through the retinohypothalamic tract to the SCN, signaling to secret glutamate in this case, and not GABA. Τhis neurotransmitter transmits to Paraventricular Nucleus (PVN), communicating with higher thoracic segments of the spinal cord and conveying information to the superior cervical ganglion. Finally, norepinephrine is released through sympathetic postsynaptic fibers and binds α1 and β1-adrenergic receptors of pinealocytes (pineal gland). When β1-adrenergic receptors are stimulated by norepinephrine signals adenylate cyclase, increasing thus, cAMP concentration and activating cAMP-dependent protein kinase. Through a cascade of signals, the transcription of arylalkylamine-N-acetyltransferase (AA-NAT) (a key enzyme in melatonin biosynthesis, see below) is induced (Figure 2) [12,14].
Melatonin (N-acetyl-5-methoxytryptamine) is synthesized and secreted mainly by the pineal gland from tryptophan. The endogenous levels of melatonin fluctuate, increasing at late afternoon, reaching a maximum concentration at around 2:00 to 4:00 and almost disappear at early morning [15]. The pineal gland is a highly vascularized, neuroendocrine organ. Tryptophan is converted intracellularly by tryptophan-5-hydroxylase and 5-hydroxytryptophan decarboxylase enzymes into serotonin. Then, serotonin is step-wise acetylated and methylated, by the actions of AA-NAT and Acetylserotonin-O-Methyltransferase (ASMT), respectively yielding melatonin [14]. In addition, multiple organs, other than pineal gland are able also to biosynthesize melatonin [12].
Melatonin binds to MT1 and MT2 melatonin GPCR receptors, resident at the SCN itself but also at peripheral oscillators (heart, intestine, eyes, vessels and other organs) [16], as shown by radiolabeled-melatonin distribution analysis [17]. Melatonin inhibits the SCN’ s multi-unit activity by acting on MT1 receptors. In general, MT1 are more widely expressed in the body than MT2, and melatonin through its binding signals mainly a decrease in intracellular cAMP [18]. In peripheral clocks, melatonin’s signaling correlates with major functions, as the circadian variation of the intraocular pressure of the eye, thermoregulation, and decrease in blood pressure [19].
Connection of peripheral clocks with SCN
The SCN is continuously connected with peripheral clocks, which are divided into four categories:
a) The autonomous pathway regulates rhythmic clock gene expression (PER1, PER2 and BMAL1), as found in the liver, through modulation of noradrenalin. SCN regulation of the peripheral clocks suggests sympathetic innervation [20].
b) Hormonal secretion is a crucial pathway for this interconnection. The corticotropin-releasing factor is secreted by the PVN, controlling the secretion of the adrenocorticotropin hormone. The latter triggers the adrenal cortex to release glucocorticoids through a signaling cascade. Glucocorticoids by binding on mineralocorticoid and glucocorticoid receptors, found in peripheral tissues, induce PER genes’ expression [4].
c) Food is also a crucial zeitgeber (external stimuli to synchronize circadian clock) of the metabolic tissues. A well-organized time window of eating affects the SCN-driven outputs and clock gene expression [4]. The optimal window between lunch and dinner is early in the afternoon, so the organism can produce melatonin, whose biosynthesis is optimal at a fasted state. Interestingly, researchers have found nucleotide polymorphisms at CLOCK to be associated with eating patterns and even metabolic disorders [21]. For instance, the ones who carry CLOCK rs4864548 haplotype are significantly associated with the appearance of metabolic syndrome [22].
d) Peripheral clock and SCN are also regulated by the differentiation of core temperature, oscillating from 36 to 37oC. The hypothalamus is responsible for regulating core body’s temperature, receiving inputs from both peripheral thermoreceptors and the SCN. Temperature homeostasis is driven via SCN feedback mechanisms modulating heat production and loss [23].
Disruption, deregulation and disorders of circadian rhythm
In modern societies, humans tend to be exposed to artificial light, at inappropriate times, particularly, at night and wake up too early in the morning. All these habits lead to short-time nocturnal sleep. Moreover, they spend a small amount of time outside, so getting enough sunlight to trigger the ipRGCs and produce melanopsin, is naturally difficult. This lack acts as a disruptive factor of the circadian rhythm [24]. Low wavelengths of 460-480nm and domestic light of 100-500 lux can suppress the secretion of melatonin at night [12,13].
As it is mentioned above, food acts as an autonomous circadian rhythm regulator directly on the body’s tissues, without the contribution of the SCN, and the random in-time eating leads again to its disruption [24]. In addition, there is the case of shift workers, who get exposed to abnormal light at night, which leads to deregulation of cortisol levels and reduces melatonin production [14]. Caffeine is a common substance for stimulating wakefulness acting by antagonizing the adenosinergic signaling [25]. Caffeine before bedtime, across healthy adults has been reported to significantly decrease the onset, amount and quality of sleep [26].
Collectively, the factors deregulating circadian clocks’ homeostasis are multiple and heterogeneous (extrinsic, social, lifestyle, behavioral and even biologic). According to the international classification of sleep disorders, CRSD are classified in six (6) categories [27], as presented in Table 1. The classification aims to distinguish each disorder towards its proper diagnosis and potent therapeutic approach.
It is intriguing to think that mutations in circadian clock genes can lead to genetic diseases, highlighting their importance/function in the proper regulation of cycle. An autosomal dominant variant of CRY1 leads to a genetic-based DSPD [28]. Mutations leading to an exon skipping lead to a dominant negative form of Cry1 that aberrantly translocates into the nucleus and inhibits the transcriptional activity of the Clock/Bmal complex. In turn, this leads to lengthening of circadian clock duration. Importantly, it is estimated that this allele affects 0.6 % of the world population.
Table 1: Presentation of CRSD along with their main characteristics.
| CRSD | Main Features |
|---|---|
Delayed sleep phase type (DSPS) |
Individuals that find difficult to fall asleep early at night and get up later in themorning |
Advanced sleep phase type |
Individuals with normal sleep-wake patterns, with too early sleep onset and offset |
Irregular sleep-wake type |
Individuals that have disorganized sleep-wake cycle |
Free running type |
Abnormal sleep-wake cycle in which sleep onset is delayed 1-2 hours due to insomnia |
Jet lag type |
Individuals who change multiple time zones and could not fall asleep and wake, accordingto the new time zone |
Shift work type |
Incapabilityto stay awake at night for work and fall asleep during the daytime |
Consequences of deregulated circadian rhythm
A disrupted circadian rhythm leads to short term discomforts that affect mood and productivity, such as lack of concentration, increased irritability, excessive daytime sleepiness, and mental fogginess. In addition, the chronic disruption of the circadian rhythm has been correlated with long term consequences [24]. To gain these results, multiple parameters should be taken into consideration, and step by step build up the big picture. In this field, animal models are mainly employed, as well as studies in the genetic cases of CRSD (referred above), in shift workers, and in those able to manage these disorders, after therapeutic intervention. In a clinical study in healthy women, simulation of the conditions of shift working correlates with obesity [29]. In fact, a recent study by Vallat R., Shah V. and Matthew Walker, conducted in a large cohort, demonstrates a strong correlation between sleep quality and glycemic status, and the common denominator is insulin sensitivity [30]. In addition, linkage and association analysis in more than 1.500 subjects demonstrated that haplotypes of BMAL1, with impaired activation activity, are associated with diabetes type 2 [31]. Estrous cycle and fertility are perhaps the most straightforward examples of deregulated system, caused by CRSD. CLOCK mutant female mice have irregular estrous cycles, and high rates of abortions, suggesting a failure by the hypothalamus to synchronize hormone secretion with daily light [32].
Pharmaceutical interventions to regulate the circadian clock
It is estimated that the prevalence of CRSD, in the general population is at 0.13-0.17%, or at 10%, according to other sources, and considered to be highly underestimated, due to mainly improper seek for medical health by the patients [33]. Moreover, there are particular fluctuations in sub-population groups, like in adolescents, where the prevalence of DSPS is between 1 to 16 % [34], in psychiatric diseases’ patients [35], and in the elderly population, frequently suffering from ASPS, attributed to a gradual decrease in melatonin by aging [36]. There is a continuous effort to study the molecular basis of CRSD. Over the years, saliva levels of melatonin are used as a relevant marker [37]. There are ongoing clinical studies with patients suffering with DSPS or ASPS, employing actigraphy and questionnaires about sleep/wake cycles (NCT00246454), or employing proteomics analysis to seek for biomarkers in patients’ plasma (NCT04690504).
The first step in treating and/or managing a CRSD is its diagnosis, related but not restricted to changes in work hours, medication(s), recent travels, evaluation of the patterns of sleep/wakefulness, categorization of the CRSD (Table 1), addiction problems, underneath physiological pathologies, fasting schedule and hereditary factors [38]. To standardize this process, questionnaires exist regarding timing and duration of sleep/wakefulness [39], enriched with questions about daytime functionality [27].
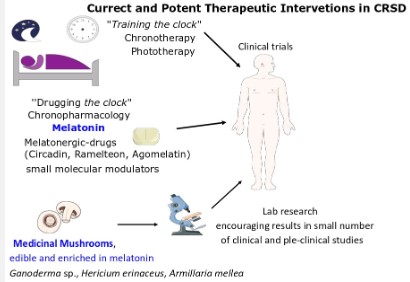
The first therapy that probably a physician will attempt with a CRSD patient is chronotherapy, working better with DSPS and ASPS patients, gradually adjusting the bedtime to the right one. Phototherapy involving exposure to artificial light sources, at the correct time also gives encouraging results in patients with CRSD. The above mentioned therapies are categorized as ‘’training the clock’’ therapies [24].
On the other hand, the ‘’drugging the clock’’ therapies or chronobiotic drugs aim to target specifically a circadian clock molecular regulator to adapt the circadian clock. Melatonin is a prototype chronobiotic drug [40], with a very wide safety window, while withdrawal effects have not be recorded [41]. Melatonin acts as the nocturnal hormone and a master orchestrator of central and peripheral circadian clocks, as described at Section 2. Oral administration of melatonin is characterized by a rapid absorption, due to its high lipophillicity, but with a bioavailability at around 15%, and a short half-life (t1/245 min) [18]. It is administered mainly to patients facing sleep problems and jet lag. An administration (0.5-5 mg) of 1-6 hours in patients suffering DSPS, free running types and work shifters, before desired sleeping time, helps in the onset of sleeping, morning alertness and quality of life [42]. The timing of administration is a key parameter, otherwise the opposite effects can be gained [43].
Agonists of MT1 and MT2 receptors (melatonergic drugs) have been developed and already approved for clinical use [44]. A slow-releasing melatonin (Circadin®) with a half-life (t1/2) of 3.5-4 h has been approved for older people (>55 years), since it was found to be more efficient for the elderly than younger people [45]. Ramelteon (t1/2 1-1.5h), is more efficient agonist for MT1 receptor than melatonin, but due to extensive first pass metabolism, it has a poor bioavailability (1.8%). It has been approved for patients suffering from delayed sleep onset, while it is highly recommended to avoid a fat diet along with its administration. Agomelatine, another agonist, binds also in MT2 and serotonergic receptors and approved for depression crisis.
The field of chronopharmacology is continually expanding as we reveal more about the molecular partners of the circadian clock. Small molecular modulators of key kinases regulating the half-life of major circadian proteins have been already discovered through Structural Activity Relationship (SAR) studies [46]. The ultimate goal is to potentially treat/manage CRSD. Future studies can shed more light on their pharmacokinetics profile, safety and efficacy of these drugs, as until now they are valuable tools for experimental and not clinical chronopharmacology.
Is there a place for clinical trials of medicinal mushrooms, rich in melatonin, to CRSDs?
There is however one more alternative, more feasible until now, to potentially manage/regulate the circadian clock, that is the use of dietary supplements of melatonin (3 mg/capsule). According to the National Sleep Foundation, the consumption of foods high in vitamin B [47] is promoted to potentially regulate melatonin levels. There are also foods (fruits, such as cherries) containing melatonin and its precursors (tryptophan and serotonin). Melatonin concertation is low (mainly <50 ng/g Dry weight) in all fruits and vegetables (see [48] for a detailed review of foods’ composition), while there is also the issue of thermal process. In LC-MS/MS analysis of pure samples of melatonin, it was found that it is partially stable for 2 h, at temperatures as high as 90oC (80% remained intact), but decreased exponentially by time thereafter [49]. Another issue to think is the poor bioavailability of orally administered melatonin, as referred above, at around 15% [50]. But still the oral administration of 2-4 mg of melatonin achieves a significant increase in its serum levels, remaining as high for at least 2 h, thereafter [37].
Μushrooms constitute a special source of melatonin and related indole compounds [48,51,52]. In Table 2 mushrooms enriched with melatonin are shown. In most cases, the same species contains also high concentrations of tryptophan and serotonin [48]. The main medicinal mushrooms are presented, along with their content in melatonin, current data (pre-clinical and clinical) in disorders, as of Central Nervous System (CNS) and cardiovascular. At last, special mention is given whether they are already exploited in the therapeutic management of CRSDs. The medicinal mushrooms are classified based on content of melatonin.
In fact, mushrooms are a valuable source of bioactive compounds (phenolic, indoles, flavonoids), unique in some cases, and thus the title of medicinal has been assigned to many of the mushrooms [73]. A large list of actions of medicinal mushrooms includes: cytotoxic, anti-inflammatory, anti-oxidative, anti-viral, antidepressive [74], as proved by pre-clinical and clinical studies, shown in Table 2. A meta-analysis in clinical studies of medicinal mushrooms, in oncology, revealed 8 clinical studies meeting the criteria, showing a merit of their in-parallel use, at the amelioration of the adverse effects of chemotherapy [75]. Medicinal mushrooms have long ago been inserted into Pharmacopoeia. Moreover, since 2011, toxicological data are required by the European Food Safety Authority (EFSA) to register a food supplement (EU Regulation No. 1924/2006). Clinical studies with medicinal mushrooms should be standardized, in terms of sample preparations, large cohort of volunteers, be placebo-controlled and blind-performed. High quality clinical studies are needed to prove clear pharmacological actions of medicinal mushrooms. To this end, EFSA recently approved mushroom powder containing vitamin D2, following the EU No. 2017/2470.
There are commercially available mushroom extracts, such as Amyloban 3399® (derived from Hericium erinaceus) traded for insomnias and sleep disorders. However, and despite most of these medicinal mushrooms being long ago in the Chinese traditional medicine, there a few clinical trials regarding their beneficial effects. To the other end, there are many such studies regarding their beneficial effects in another conditions (see [73] for a detailed review of these clinical trials). In a recent placebocontrolled clinical study 77 overweight volunteers facing sleep disorders were administered extracts from Hericium erinaceus [69]. Both their mood and sleep performance were improved by the mushroom extract administration, for 2 months as evaluated, via self-scores. In a second clinical trial published in 2015, the extracts of the same medicinal mushroom has been tested in a small sample (8 undergraduate students) receiving blinded, questionnaire [68]. According to the authors, the anxiety of the volunteers was decreased but they were inconclusive for the sleep quality, since a larger cohort was needed.
There are clinical trials with these medicinal mushrooms testing in other CNS disorders, as depression. In a double-blinded, placebo controlled study, the extracts of the same medicinal mushroom were tested for an entire year, in 49 patients with mild Alzheimer’s disease [67], (NCT04065061). Magnetic Resonance Imaging depicted better visual contrast sensitivity in those patient who received the extract, indicative of less amyloid plaques formation. Moreover, illness-related fatigue was reduced in a placebo-control and double blinded clinical trial, in 48 patients, treated with hormone therapy to fight breast cancer [61]. In this case, extracts of Ganoderma lucidum were used, recording also no obstacles in the main treatment scheme and no toxicity, as assessed by biochemical analysis.
In delineated, pre-clinical studies (in rats), the extract of Armillaria mellea (used in China as a traditional drug for insomnia) increased sleep duration and decreased sleep latency, in chlorophenylalanine-induced insomnia. In this case, elevated serum levels of antioxidants, as GSH peroxidase were recorded [72]. The extract of Ganoderma lucidum increased total sleep time in rats, as recorded via the SleepSign® software [62]. The authors concluded that the extract acts as hypnotic, via a mechanism partially dependent on interleukins’ action. In addition, the patented Jerte Valley cherry-based nutraceutical product, rich in melatonin and its precursors, were administered in experimental rats and ringdoves, representative as nocturnal and diurnal animals, respectively [76]. Both young and older animals, having lower endogenous melatonin levels, were used. The treatment increased serum melatonin levels in the animals, irrespective of age, and their motor activity at night (for rats) and day (for ringdoves).
Table 2: Medicinal mushrooms with high content in melatonin.
| Mushroom species | Composition in Melatonin (mg/100 g dry weight) | Status in other diseases | Status in CRSD |
|---|---|---|---|
| Cantharellus cibarious | 0.11 [53] | In animals-anti-inflammatory, wound healing [54] | Not found |
| Agaricus bisporus | 0.43-0.64 [55] | Clinical trial for cardiovascular Diseases (NCT04257201) | Not found |
| Boletus edulis | 0.68 [56] | In vitro-Gastrointestinal [57] In vitro-antioxidant properties [58] |
Not found |
| Suillus luteus | 0.71 [56] | In vitro-anti-proliferative [59] | Not found |
| Ganoderma lingzhi (Reishi Mushrooms) Ganoderma lucidum | 0.02-0.98 (Ganoderma spp.) [60] | Clinical Trial Fatigue in hormone therapy cancer patients [61] | Mushroom extracts in trademarket, traditional Chinese medicine for insomnias, In animals, sleep disorder [62], anti-insomnia, In silico [63] |
| Hericium erinaceus (Lion’s Mane Mushroom) | 1 [64] | Depressive disorder Reviews [65, 66] Clinical Trial Alzheimer’s disease (NCT04065061) [67] | Mushroom extracts in trademarket Clinical Trials_Sleep Disorders [68, 69] |
| Lactarius deliciosus | 1.29 [48] | in vitro-antioxidant and anti-hyperglycemic [70]. Depression [66] | Not found |
| Armillaria mellea | 22.9 [48] | In animals, memory [71] | Mushroom extract in trademarket Traditional Chinese medicine for insomnias in animals, sleep disorder [72] |
Future perspectives
One third of the world population is affected by insomnia [48], while we are still learning about the negative impact(s) of chronic insomnia and CRSD on body’s homeostasis. It is intriguing to think that CLOCK mutants in mice, where compensatory mechanisms still act to support the circadian rhythm, impaired quality of life and decreased total lifespan by 15% [77]. Accumulating clinical and pre-clinical evidence indicates the potential of medicinal mushrooms, rich in serotonin, as a therapeutic intervention, in depressive disorders [66]. As analyzed above, the trials of medicinal mushrooms in CRSD, though encouraging, remain limited. In an in silico analysis of the anti-insomnia effects of Ganoderma sp., multiple ingredients, including melatonin precursors, are implicated in their medicinal effects [63]. The beneficial effects of melatonin in human health are multiple, among them its well-established antioxidant effect [78]. In Figure 3, the current and potential therapeutic approaches of CRSDs are summarized.
Further studies of medicinal mushrooms in the management of CRSD are substantiated given the fact that the concentration in melatonin is close to therapeutic levels. Most of these medicinal mushrooms are edible, known not to contain toxic ingredients, as the Lactarius deliciosus cultivated in the Mediterranean area. However, major caveats need to be properly addressed including detailed toxicology assessment. Moreover, any potential drug interactions should be taken into account, based on the multiple ingredients of medicinal mushrooms. The available food supplements containing also GABA agonists, enhance the need for a central regulatory control of such products. The preparation of medicinal mushrooms’ extracts should take into account the variations in melatonin composition due to stressinduction [79]. There is also the experience from artificial digestive juices of medicinal mushrooms helping the extraction of ingredients [80].
Conclusion
Management of the deregulations of circadian clock is a rather complicated issue and interrelated to multiple mechanisms. As modern medicine tends to move to a more circadian clock-centered approach, the potential use of edible medicinal mushrooms must be considered through better organized preclinical and clinical studies.
Problem statement
Modern lifestyle significantly impairs the orchestration of circadian clock and millions of people worldwide are affected. The administration of melatonin is the gold standard in ‘’drugging the clock’’ therapeutic approaches. On the other hand, medicinal mushrooms are used in traditional medicine for insomnia. Medicinal, edible mushrooms are already proposed for management of depressive disorders, while a few, encouraging pre-clinical and clinical data exist with Circadian Rhythm Sleep Disorders (CRSD). In the present review, these studies are analyzed, and future perspectives to increase the scientific attendance on this issue and get over the obstacles are discussed.
Declarations
Conflict of interest disclosure: The authors declare have no conflict of interest.
Acknowledgment: We want to thank Dr. Elena Siapati and Dr. Elena Barda for their constructive comments on the manuscript.
References
- Kramer A, et al. Foundations of circadian medicine. PLoS Biol. 2022; 20: e3001567.
- Huang RC. The discoveries of molecular mechanisms for the circadian rhythm: The 2017 Nobel Prize in Physiology or Medicine. Biomed J. 2018; 41: 5-8.
- Koronowski KB, Sassone-Corsi P. Communicating clocks shape circadian homeostasis. Science. 2021; 371.
- de Assis LVM, H Oster. The circadian clock and metabolic homeostasis: Entangled networks. Cell Mol Life Sci. 2021; 78: 4563-4587.
- Zhang R, et al. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci USA. 2014; 111: 16219-24.
- Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012; 338: 349-54.
- Sassone-Corsi P. The Epigenetic and Metabolic Language of the Circadian Clock, in A Time for Metabolism and Hormones, P. Sassone-Corsi and Y. Christen, Editors. 2016: Cham (CH). 2016; 1-11.
- Menet JS, et al. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. eLife. 2012; 1: e00011.
- Cheng HY, et al. microRNA modulation of circadian-clock period and entrainment. Neuron, 2007; 54: 813-29.
- Robles MS, SJ Humphrey, M Mann. Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell Metab. 2017; 25: 118-127.
- Hughes S, et al. Signalling by melanopsin (OPN4) expressing photosensitive retinal ganglion cells. Eye (Lond). 2016; 30: 247- 54.
- Arendt J, A Aulinas. Physiology of the Pineal Gland and Melatonin, in Endotext, KR. Feingold, et al. Editors. 2000: South Dartmouth (MA). 2000.
- Higuchi S, et al. Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab. 2014; 99: 3298-303.
- Vasey C, J McBride, K Penta. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients. 2021; 13.
- Dujardin S, A Pijpers, D Pevernagie. Prescription Drugs Used in Insomnia. Sleep Med Clin. 2020; 15: 133-145.
- Pevet P. Melatonin receptors as therapeutic targets in the suprachiasmatic nucleus. Expert Opin Ther Targets. 2016; 20: 1209- 18.
- Morgan PJ, et al. Melatonin receptors: Localization, molecular pharmacology and physiological significance. Neurochem Int. 1994; 24: 101-46.
- Pandi-Perumal SR, et al. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008; 85: 335-53.
- Viswanathan M, JT Laitinen, JM Saavedra. Expression of melatonin receptors in arteries involved in thermoregulation. Proc Natl Acad Sci U S A. 1990; 87: 6200-3.
- Terazono H, et al. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci U S A. 2003; 100: 6795-800.
- Froy O, M Garaulet. The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr Rev. 2018; 39: 261-273.
- Scott EM, AM Carter, PJ Grant. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond). 2008; 32: 658-62.
- Webster WW, B Smarr. Using Circadian Rhythm Patterns of Continuous Core Body Temperature to Improve Fertility and Pregnancy Planning. J Circadian Rhythms. 2020; 18: 5.
- Sulli G, et al. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol Sci. 2018; 39: 812-827.
- Reichert CF, T Deboer, HP Landolt. Adenosine, caffeine, and sleep-wake regulation: State of the science and perspectives. J Sleep Res. 2022; 31: e13597.
- Gardiner C, et al. The effect of caffeine on subsequent sleep: A systematic review and meta-analysis. Sleep Med Rev. 2023; 69: 101764.
- Kim MJ, JH Lee, JF Duffy. Circadian Rhythm Sleep Disorders. J Clin Outcomes Manag. 2013; 20: 513-528.
- Patke A, et al. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell. 2017; 169: 203-215 e13.
- McHill AW, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A. 2014; 111: 17302-7.
- Vallat R, VD Shah, MP Walker. Coordinated human sleeping brainwaves map peripheral body glucose homeostasis. Cell Rep Med. 2023; 4: 101100.
- Woon PY, et al. Aryl hydrocarbon receptor nuclear translocatorlike (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007; 104: 14412- 7.
- Miller BH, et al. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004; 14: 1367-73.
- Karna B, A Sankari, G Tatikonda. Sleep Disorder, in StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Abdulghani Sankari declares no relevant financial relationships with ineligible companies. Disclosure: Geethika Tatikonda declares no relevant financial relationships with ineligible companies. 2023.
- Gradisar M, SJ Crowley. Delayed sleep phase disorder in youth. Curr Opin Psychiatry. 2013; 26: 580-5
- Takaesu Y, et al. Prevalence of Circadian Rhythm Sleep-Wake Disorders and Associated Factors in Euthymic Patients with Bipolar Disorder. PLoS One. 2016; 11: e0159578.
- Wurtman RJ. Age-related decreases in melatonin secretion-clinical consequences. J Clin Endocrinol Metab. 2000; 85: 2135-6.
- Shirakawa S, et al. Time course of saliva and serum melatonin levels after ingestion of melatonin. Psychiatry Clin Neurosci. 1998; 52: 266-7.
- Gamaldo, RESACE. Diagnostic and therapeutic considerations in sleep disorders case studies and commentary. Journal of Clinical Outcomes Management. 2011; 18: 129-144.
- Roenneberg T, A Wirz-Justice, M Merrow. Life between Clocks: Daily Temporal Patterns of Human Chronotypes. Journal of Biological Rhythms. 2003; 18: 80-90.
- Starkey SJ, et al. Modulation of the rat suprachiasmatic circadian clock by melatonin in vitro. Neuroreport. 1995; 6: 1947-51.
- Stone BM, et al. Hypnotic activity of melatonin. Sleep. 2000; 23: 663-9.
- Riemersma-van der Lek RF, et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008; 299: 2642-55.
- Arendt J. Approaches to the Pharmacological Management of Jet Lag. Drugs. 2018; 78: 1419-1431.
- Kim HK, KI Yang. Melatonin and melatonergic drugs in sleep disorders. Transl Clin Pharmacol. 2022; 30: 163-171.
- Wade AG, et al. Prolonged release melatonin in the treatment of primary insomnia: evaluation of the age cut-off for short- and long-term response. Curr Med Res Opin. 2011; 27: 87-98.
- Miller S, T Hirota. Pharmacological Interventions to Circadian Clocks and Their Molecular Bases. J Mol Biol. 2020; 432: 3498- 3514.
- Djokic G, et al. The Effects of Magnesium - Melatonin - Vit B Complex Supplementation in Treatment of Insomnia. Open Access Maced J Med Sci. 2019; 7: 3101-3105.
- Meng X, et al. Dietary Sources and Bioactivities of Melatonin. Nutrients. 2017; 9.
- Pranil T, A Moongngarm, P Loypimai. Influence of pH, temperature, and light on the stability of melatonin in aqueous solutions and fruit juices. Heliyon. 2020; 6: e03648.
- DeMuro RL, et al. The absolute bioavailability of oral melatonin. J Clin Pharmacol. 2000; 40: 781-4.
- Podkowa A, et al. Culinary-medicinal mushrooms: A review of organic compounds and bioelements with antioxidant activity. European Food Research and Technology. 2021; 247: 513-533.
- Muszyńska B, et al. TLC-UV Analysis of Indole Compounds and other Nitrogen-Containing Bases in the Fruiting Bodies of Lactarius deterrimus. JPC-Journal of Planar Chromatography- Modern TLC. 2007; 20: 57-60.
- Muszynska B, K Sulkowska-Ziaja, H Ekiert. Analysis of indole compounds in methanolic extracts from the fruiting bodies of Cantharellus cibarius (the Chanterelle) and from the mycelium of this species cultured in vitro. J Food Sci Technol. 2013; 50: 1233-7.
- Nasiry D, AR Khalatbary, MA Ebrahimzadeh. Anti-Inflammatory and Wound-Healing Potential of Golden Chanterelle Mushroom, Cantharellus cibarius (Agaricomycetes). Int J Med Mushrooms. 2017; 19: 893-903.
- Muszynska B, et al. Agaricus bisporus and its in vitro culture as a source of indole compounds released into artificial digestive juices. Food Chem. 2016; 199: 509-15.
- Muszynska B, K Sutkowska-Ziaja, H Ekiert. Indole compounds in some culinary-medicinal higher basidiomycetes from Poland. Int J Med Mushrooms. 2011; 13: 449-54.
- Avram I, et al. Boletus edulis Extract-A New Modulator of Dysbiotic Microbiota. Life (Basel). 2023; 13.
- Witkowska AM, ME Zujko, I Mironczuk-Chodakowska. Comparative study of wild edible mushrooms as sources of antioxidants. Int J Med Mushrooms. 2011; 13: 335-41.
- Aytar E, I Akata, L Açık. Antioxidant, Antimicrobial And Anti-Proliferative Activity Of Suillus Luteus (L.) Roussel Extracts Suillus Luteus (L).Roussel Ekstresi’nin Antioksidan, Antimikrobiyal Ve Anti-Proliferatif Aktivitesi. Ankara Universitesi Eczacilik Fakultesi Dergisi. 2020; 44: 374.
- Sulkowska-Ziaja K, et al. Bioactivity and Mycochemical Profile of Extracts from Mycelial Cultures of Ganoderma spp. Molecules. 2022; 27.
- Zhao H, et al. Spore Powder of Ganoderma lucidum Improves Cancer-Related Fatigue in Breast Cancer Patients Undergoing Endocrine Therapy: A Pilot Clinical Trial. Evid Based Complement Alternat Med. 2012; 2012: 809614.
- Cui XY, et al. Extract of Ganoderma lucidum prolongs sleep time in rats. J Ethnopharmacol. 2012; 139: 796-800.
- Qiu Y, et al. Exploration of the anti-insomnia mechanism of Ganoderma by central-peripheral multi-level interaction network analysis. BMC Microbiol. 2021; 21: 296.
- Włodarczyk A, FA Jędrejko K, Zięba P, Lazur J, Sułkowska-Ziaja K, et al. Edible and medicinal mushroom Hericium erinaceus as a potential natural material with influence on brain functions. 2020; 29: 4-0.
- Chong PS, et al. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int J Mol Sci. 2019; 21.
- Fijalkowska A, et al. Edible Mushrooms as a Potential Component of Dietary Interventions for Major Depressive Disorder. Foods. 2022; 11.
- Li IC, et al. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study. Front Aging Neurosci. 2020; 12: 155.
- Okamura H, et al. The effects of Hericium erinaceus (Amyloban® 3399) on sleep quality and subjective well-being among female undergraduate students: A pilot study. Personalized Medicine Universe. 2015; 4: 76-78.
- Vigna L, et al. Hericium erinaceus Improves Mood and Sleep Disorders in Patients Affected by Overweight or Obesity: Could Circulating Pro-BDNF and BDNF Be Potential Biomarkers? Evid Based Complement Alternat Med. 2019; 2019: 7861297.
- Xu Z, et al. Chemical Composition, Antioxidant and Antihyperglycemic Activities of the Wild Lactarius deliciosus from China. Molecules. 2019; 24.
- Li H, G Xu, G Yuan. Effects of an Armillaria mellea Polysaccharide on Learning and Memory of D-Galactose-Induced Aging Mice. Front Pharmacol. 2022; 13: 919920.
- Yao L, et al. Armillaria mellea fermentation liquor ameliorates p-chlorophenylalanine-induced insomnia associated with the modulation of serotonergic system and gut microbiota in rats. J Food Biochem. 2022; 46: e14075.
- Venturella G, et al. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int J Mol Sci. 2021; 22.
- Rezic Muzinic N, et al. Golden Chanterelle or a Gold Mine? Metabolites from Aqueous Extracts of Golden Chanterelle (Cantharellus cibarius) and Their Antioxidant and Cytotoxic Activities. Molecules. 2023; 28.
- Jeitler M, et al. Significance of Medicinal Mushrooms in Integrative Oncology: A Narrative Review. Front Pharmacol. 2020; 11: 580656.
- Delgado J, et al. Diets enriched with a Jerte Valley cherry-based nutraceutical product reinforce nocturnal behaviour in young and old animals of nocturnal (Rattus norvegicus) and diurnal (Streptopelia risoria) chronotypes. J Anim Physiol Anim Nutr (Berl). 2013; 97: 137-45.
- Dubrovsky YV, WE Samsa, RV Kondratov. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY). 2010; 2: 936-44.
- Korkmaz A, et al. Melatonin: An established antioxidant worthy of use in clinical trials. Mol Med. 2009; 15: 43-50.
- Tan DX, et al. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules. 2015; 20: 18886-906.
- Kała K, et al. Kinetics of extracted bioactive components from mushrooms in artificial digestive juices. International Journal of Food Properties. 2017; 20: 1796-1817.