Short Commentary
Volume 2, Issue 7
On the Mechanism of Alcoholysis between Fatty Acid Methyl Esters and Trimethylolpropane
Ilona S Kozeeva*; Valentin N Sapunov; Michial S Voronov
Mendeleev University of Chemical Technology of Russia, Russia.
Corresponding Author:
Ilona S Kozeeva
Email: iolanta2006@list.ru
Received : Jun 29, 2023 Accepted : Jul 14, 2023 Published : Jul 21, 2023 Archived : www.meddiscoveries.org
Citation: Kozeeva IS, Sapunov VN, Voronov MS. On the Mechanism of Alcoholysis between Fatty Acid Methyl Esters and Trimethylolpropane. Med Discoveries. 2023; 2(7): 1056.
Copyright: © 2023 Kozeeva IS. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This paper reports on a previously undescribed mechanism of the alcoholysis of fatty acid methyl esters with a polyol, in particular, with trimethylolpropane. The chromatographic data of the reaction products are illustrated by calculations of the mass and molar concentrations of all participating components of the system. Some observable phenomena have been noted. The authors propose an extended mechanism of the alcoholysis process.
Keywords: Mechanism; Alcoholysis; Chromatogram; Trimethylolpropane; Fatty acid methyl ester.
Introduction
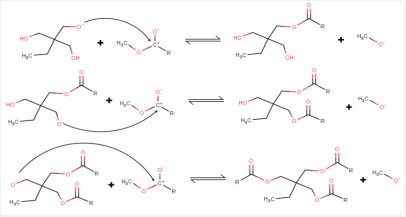
The mechanism of the alkylation of FAME by with trimethylolpropane (TMP) to give mono, di, and tri-substituted TMPs (METMP, DETMP и TETMP, respectively) is usually referred to as a series of reversible sequentially parallel reactions, considering it to be “classical” [1-5] (Scheme 1). A significant draw-back of the proposed mechanism is its inability to correctly reflect the experimental data. There is also a contradiction with real events, such as the reaction going on despite a serious shortage of raw materials.
Experimental section
To find out how incomplete TMP esters might interact with one another, several synthesizes were performed. The first stage of syntheses was place over the course of a two-hour period at a temperature of 120°C, atmospheric pressure, and a catalytic quantity of KOH with a molar ratio of 1:3 between TMP and FAME. Chromatographic analysis was used to examine the syntheses' outcomes. The reaction mass was analyzed using gas capillary chromatography (SGE HT5, 12 m x 0.53 mm, inner diameter 0.15 m, fitted with a GC-FID (flame ionization detector). The silylation procedure was used to prepare the sample. To a vial with a 10 mL capacity, 30 ± 5 mg of ester must be added. The mixture must then be diluted with 1 mL of GC-grade ethyl acetate. The combination was dissolved by swirling the sample for a while. The mixture was then given a 1 ml addition of BSTFA reagent. The vial was then moved to a water bath maintained at 40°C and allowed to sit there for 10 minutes. According to Yunus et al [6], the GC parameters were applied. The column temperature was initially set at 50°C, held steady for 5 min, and then increased to 340°C at a rate of 5°C/min. The injector and detector temperatures were set at 300 and 360°C, respectively. Hydrogen, the GC system's carrier gas, was introduced at a flowrate of 25 mL/min. One microliter of material was injected into the GC apparatus, with the split ratio set to 1:1. By comparing the retention times to real standards, the peaks were found.
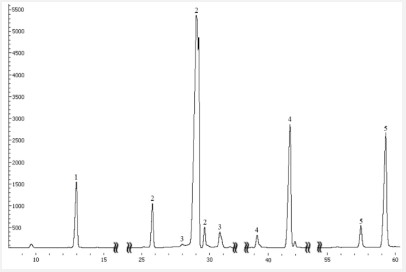
The typical chromatogram of reaction mass illustrated corresponds to the synthesis described above (Figure 1).
According to the results of chromatographic analysis, it was found that 2 hours after the start of synthesis, TMP was practically used up, while the FAME content decreased by 30%, the TMP conversion was 74%, the FAME conversion was 36%, the total content of esters was over 40% by weight.
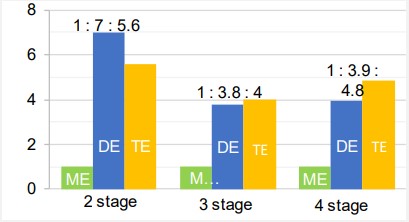
At the second stage, the reaction mass was purified from the initial substances (TMP residues and a significant amount of FAMEs) by short path vacuum distillation apparatus at 170°С for 1 hour and residual pressure less 0.5 mbar. For further research, a distillation residue containing mono-, di- and triesters, as well as residual amounts of trimethylolpropane and methyl esters was used (Table 1). Then the resulting product was again analyzed by GC. Change in the ratio of mono-, di- and triesters of TMP and fatty acids (METMP, DETMP and TETMP, respectively) in the final product is noted.
At the 3 and 4 stages, the distillation residue was again held at a temperature of 120°C for 60 and 200 minutes, respectively, with stirring and methanol removal. Then the composition was analyzed by GC (Table 1).
Table 1: Mass composition stagy-by-stage.
| TMP | FAME | METMP | DETMP | TETMP | |
|---|---|---|---|---|---|
| Start | 13.0 | 87.0 | 0 | 0 | 0 |
| Stage 1 | 3.2 | 55.0 | 1.6 | 19.0 | 21.2 |
| Stage 2 | 0.4 | 11.5 | 5.3 | 33.5 | 49.2 |
| Stage 3 | 1.6 | 10.9 | 4.7 | 30.5 | 52.3 |
| Stage 4 | 2.6 | 8.5 | 7.2 | 28.9 | 52.8 |
According to the analyzes results of samples after distillation purification, an increase in the content of TMP, further consumption of FAME residues, as well as the formation and redistribution of the ratio of mono-, di-, and triesters were observed.
Results and discussion
According to the chromatographic analysis, it was found that the process of alcoholysis continues even with a significant deviation of the starting reagents from the stoichiometric. Due to the equilibrium type of reactions, the synthesis should have stopped or significantly slowed down after distillation of trimethylolpropane and methyl esters of fatty acids, since the direct reaction would be suppressed by an excess of reaction products.
In addition, during the synthesis, when passing from stage 2 to stage 3 and 4, the accumulation of TMP was observed when the residual amounts of FAME were consumed. The classical mechanism suggests that the formation of TMP is possible only because of a single reverse reaction between ME and methanol. However, the experiment was organized in such a way that methanol was effectively removed from the reaction mass immediately after its formation. This would prevent the reverse reaction between ME and methanol from proceeding and would not allow TMP to accumulate. Therefore, the accumulation of TMP must have other causes.
Additionally, during the experiments, a slight release of methanol and accumulation of METMP were observed, which is consistent with the consumption of FAMEs.
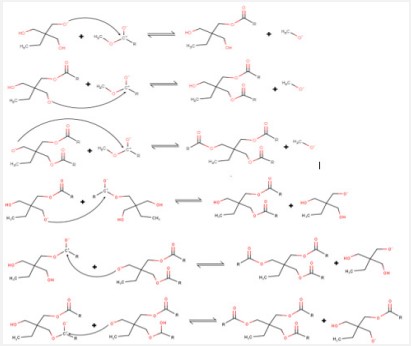
As noted above, an assumption was made that the classical known mechanism cannot fully describe the ongoing process. The authors propose an augmented reaction scheme (Scheme 2 below). The augmented mechanism is based on the idea of interactions and interconversions of intermediate synthesis products. It is assumed that the resulting METMP and DETMP of trimethylolpropane interact with each other in various ways, with the formation of target key products and trimethylolpropane.
Our assumptions are confirmed by the additional formation of TMP with concomitant consumption of residual FAME (Table 1).
The bimolecular reaction of ME or DE and the interactions between ME and DE is consistent with the obtained ester molar ratio redistribution data (Figure 2).
The redistribution of the target products indicates that the reaction continued in the absence of TMP. The observed increase in the relative content of METMP could not be explained by the "classical" mechanism, according to which TMP is necessary for the formation of a monoester. This phenomenon also confirms the idea proposed by the authors about the insufficiency of the existing mechanism.
The proposed supplemented mechanism makes it possible to clarify the nature of the observed features of the process the alcoholysis of FAMEs by TMP.
Additional research is the goal of future work.
Declarations
Associated content: This material is available free of charge via the Internet at http://pubs.acs.org.
Author informations
Valentin N. Sapunov: Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, 125047, Moscow, Russia; orcid.org/0000-0002-1006-5436
Michail S. Voronov: Mendeleev University of Chemical Technology of Russia, Miusskaya square, 9, 125047, Moscow, Russia; orcid.org/0000-0001-7839-6250
Acknowledgment: The work is performed in the framework of the development program “Priority-2030” of the Mendeleev University of Chemical Technology of Russia
Abbreviations: TMP: Trimethylolpropane; FAME: Fatty acid methyl ester; ME: Monosaturated ester fatty acid and trimethylolpropane; DE: Disaturated ester fatty acid and trimethylolpropane; TE: Trisaturated ester fatty acid and trimethylolpropane; GC: Gas chromatography.
References
- Yunus R, Fakhru’I-Razi A, Ooi TL. et al. Kinetics of transesterification of palm-based methyl es-ters with trimethylolpropane. J Amer Oil Chem Soc. 2004; 81: 497–503.
- Abd Hamid, Hamidah, Robiah Yunus, and Thomas SY Choong. Utilization of MATLAB to sim-ulate kinetics of transesterification of palm oil-based methyl esters with trimethylolpropane for biode-gradable synthetic lubricant synthesis. Chemical Product and Process Modeling. 2010; 5.1.
- Menkiti, Matthew, et al. Process optimization and kinetics of biolubricant synthesis from fluted pumpkin seed. Journal of Chemical Engineering. 2015; 11: 1857-7881.
- Resul, Mohamad Faiz Mukhtar Gunam, Tinia Idaty Mohd Ghazi, and Azni Idris. Kinetic study of jatropha biolubricant from transesterification of jatropha curcas oil with trimethylolpropane: Effects of temperature. Industrial Crops and Products. 2012; 38: 87-92.
- Menkiti, Matthew, et al. Process optimization and kinetics of biolubricant synthesis from fluted pumpkin seed. Journal of Chemical Engineering. 2015; 11: 1857-7881.
- Yunus, Robiah, et al. A simple capillary column GC method for analysis of palm oil-based polyol esters. Journal of the American Oil Chemists’ Society. 2002; 79: 1075-1080.